Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations
- PMID: 20729831
- PMCID: PMC3129007
- DOI: 10.1038/nature09327
Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations
Abstract
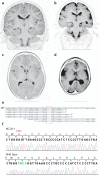
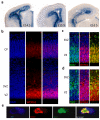
The development of the human cerebral cortex is an orchestrated process involving the generation of neural progenitors in the periventricular germinal zones, cell proliferation characterized by symmetric and asymmetric mitoses, followed by migration of post-mitotic neurons to their final destinations in six highly ordered, functionally specialized layers. An understanding of the molecular mechanisms guiding these intricate processes is in its infancy, substantially driven by the discovery of rare mutations that cause malformations of cortical development. Mapping of disease loci in putative Mendelian forms of malformations of cortical development has been hindered by marked locus heterogeneity, small kindred sizes and diagnostic classifications that may not reflect molecular pathogenesis. Here we demonstrate the use of whole-exome sequencing to overcome these obstacles by identifying recessive mutations in WD repeat domain 62 (WDR62) as the cause of a wide spectrum of severe cerebral cortical malformations including microcephaly, pachygyria with cortical thickening as well as hypoplasia of the corpus callosum. Some patients with mutations in WDR62 had evidence of additional abnormalities including lissencephaly, schizencephaly, polymicrogyria and, in one instance, cerebellar hypoplasia, all traits traditionally regarded as distinct entities. In mice and humans, WDR62 transcripts and protein are enriched in neural progenitors within the ventricular and subventricular zones. Expression of WDR62 in the neocortex is transient, spanning the period of embryonic neurogenesis. Unlike other known microcephaly genes, WDR62 does not apparently associate with centrosomes and is predominantly nuclear in localization. These findings unify previously disparate aspects of cerebral cortical development and highlight the use of whole-exome sequencing to identify disease loci in settings in which traditional methods have proved challenging.
Figures


Comment in
-
Whole-exome sequencing: a powerful technique for identifying novel genes of complex disorders.Clin Genet. 2011 Feb;79(2):132-3. doi: 10.1111/j.1399-0004.2010.01585.x. Epub 2010 Nov 19. Clin Genet. 2011. PMID: 21087231 No abstract available.
References
-
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–22. - PubMed
-
- Guerrini R, Dobyns WB, Barkovich AJ. Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options. Trends Neurosci. 2008;31:154–62. - PubMed
-
- Guerrini R. Genetic malformations of the cerebral cortex and epilepsy. Epilepsia. 2005;46(Suppl 1):32–7. - PubMed
-
- Guerrini R, Carrozzo R. Epilepsy and genetic malformations of the cerebral cortex. Am J Med Genet. 2001;106:160–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

