Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells
- PMID: 20729850
- PMCID: PMC3388774
- DOI: 10.1038/nmat2812
Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells
Abstract
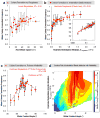
Both human embryonic stem cells and induced pluripotent stem cells can self-renew indefinitely in culture; however, present methods to clonally grow them are inefficient and poorly defined for genetic manipulation and therapeutic purposes. Here we develop the first chemically defined, xeno-free, feeder-free synthetic substrates to support robust self-renewal of fully dissociated human embryonic stem and induced pluripotent stem cells. Material properties including wettability, surface topography, surface chemistry and indentation elastic modulus of all polymeric substrates were quantified using high-throughput methods to develop structure-function relationships between material properties and biological performance. These analyses show that optimal human embryonic stem cell substrates are generated from monomers with high acrylate content, have a moderate wettability and employ integrin alpha(v)beta(3) and alpha(v)beta(5) engagement with adsorbed vitronectin to promote colony formation. The structure-function methodology employed herein provides a general framework for the combinatorial development of synthetic substrates for stem cell culture.
Figures





References
-
- Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

