Cell adhesion: integrating cytoskeletal dynamics and cellular tension
- PMID: 20729930
- PMCID: PMC2992881
- DOI: 10.1038/nrm2957
Cell adhesion: integrating cytoskeletal dynamics and cellular tension
Abstract
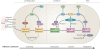
Cell migration affects all morphogenetic processes and contributes to numerous diseases, including cancer and cardiovascular disease. For most cells in most environments, movement begins with protrusion of the cell membrane followed by the formation of new adhesions at the cell front that link the actin cytoskeleton to the substratum, generation of traction forces that move the cell forwards and disassembly of adhesions at the cell rear. Adhesion formation and disassembly drive the migration cycle by activating Rho GTPases, which in turn regulate actin polymerization and myosin II activity, and therefore adhesion dynamics.
Figures



References
-
- Campbell ID, Ginsberg MH. The talin–tail interaction places integrin activation on FERM ground. Trends Biochem. Sci. 2004;29:429–435. - PubMed
-
- Premont RT. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell. Signal. 2004;16:1001–1011. - PubMed
-
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nature Rev. Mol. Cell Biol. 2009;10:21–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

