Mitochondrial stress and the pathogenesis of diabetic neuropathy
- PMID: 20729997
- PMCID: PMC2924887
- DOI: 10.1586/eem.09.55
Mitochondrial stress and the pathogenesis of diabetic neuropathy
Abstract
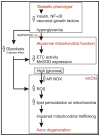
Diabetic neuropathy is a major complication of diabetes that affects the sensory and autonomic nervous systems and leads to significant morbidity and impact on quality of life of patients. Mitochondrial stress has been proposed as a major mediator of neurodegeneration in diabetes. This review briefly summarizes the nature of sensory and autonomic nerve dysfunction and presents these findings in the context of diabetes-induced nerve degeneration mediated by alterations in mitochondrial ultrastructure, physiology and trafficking. Diabetes-induced dysfunction in calcium homeostasis is discussed at length and causative associations with sub-optimal mitochondrial physiology are developed. It is clear that across a range of complications of diabetes that mitochondrial physiology is impaired, in general a reduction in electron transport chain capability is apparent. This abnormal activity may predispose mitochondria to generate elevated reactive oxygen species (ROS), although experimental proof remains lacking, but more importantly will deleteriously alter the bioenergetic status of neurons. It is proposed that the next five years of research should focus on identifying changes in mitochondrial phenotype and associated cellular impact, identifying sources of ROS in neurons and analyzing mitochondrial trafficking under diabetic conditions.
Figures
Similar articles
-
Mechanisms of disease: Mitochondrial dysfunction in sensory neuropathy and other complications in diabetes.Handb Clin Neurol. 2014;126:353-77. doi: 10.1016/B978-0-444-53480-4.00027-8. Handb Clin Neurol. 2014. PMID: 25410234 Review.
-
Oxidative Stress and Mitochondrial Dysfunction Associated with Peripheral Neuropathy in Type 1 Diabetes.Antioxid Redox Signal. 2022 Sep;37(7-9):578-596. doi: 10.1089/ars.2021.0152. Epub 2021 Dec 7. Antioxid Redox Signal. 2022. PMID: 34416846 Review.
-
The role of aberrant mitochondrial bioenergetics in diabetic neuropathy.Neurobiol Dis. 2013 Mar;51:56-65. doi: 10.1016/j.nbd.2012.03.016. Epub 2012 Mar 9. Neurobiol Dis. 2013. PMID: 22446165 Review.
-
Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats.Diabetes. 2011 Jan;60(1):288-97. doi: 10.2337/db10-0818. Epub 2010 Sep 28. Diabetes. 2011. PMID: 20876714 Free PMC article.
-
Mechanism of mitochondrial dysfunction in diabetic sensory neuropathy.J Peripher Nerv Syst. 2003 Dec;8(4):227-35. doi: 10.1111/j.1085-9489.2003.03028.x. J Peripher Nerv Syst. 2003. PMID: 14641647
Cited by
-
Mitochondria in the pathogenesis of diabetes: a proteomic view.Protein Cell. 2012 Sep;3(9):648-60. doi: 10.1007/s13238-012-2043-4. Epub 2012 Jun 22. Protein Cell. 2012. PMID: 22729395 Free PMC article. Review.
-
Synaptic transmission at parasympathetic neurons of the major pelvic ganglion from normal and diabetic male mice.J Neurophysiol. 2013 Feb;109(4):988-95. doi: 10.1152/jn.00354.2012. Epub 2012 Nov 28. J Neurophysiol. 2013. PMID: 23197460 Free PMC article.
-
Mitotoxicity in distal symmetrical sensory peripheral neuropathies.Nat Rev Neurol. 2014 Jun;10(6):326-36. doi: 10.1038/nrneurol.2014.77. Epub 2014 May 20. Nat Rev Neurol. 2014. PMID: 24840972 Free PMC article. Review.
-
Three-dimensional Imaging and Analysis of Mitochondria within Human Intraepidermal Nerve Fibers.J Vis Exp. 2017 Sep 29;(127):53369. doi: 10.3791/53369. J Vis Exp. 2017. PMID: 28994751 Free PMC article.
-
Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy.Neuron. 2013 Mar 6;77(5):886-98. doi: 10.1016/j.neuron.2013.01.012. Neuron. 2013. PMID: 23473319 Free PMC article.
References
-
- Malik RA, Tesfaye S, Newrick PG, et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48(3):578–585. - PubMed
-
- Sima AA. Diabetic neuropathy in type 1 and type 2 diabetes and the effects of C-peptide. J Neurol Sci. 2004;220(1–2):133–136. - PubMed
-
- Yagihashi S. Pathogenetic mechanisms of diabetic neuropathy: lessons from animal models. J Peripher Nerv Syst. 1997;2(2):113–132. - PubMed
-
- Toth C, Brussee V, Cheng C, Zochodne DW. Diabetes mellitus and the sensory neuron. J Neuropathol Exp Neurol. 2004;63(6):561–573. - PubMed
-
- Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
