Strong Circularly Polarized Luminescence from Highly Emissive Terbium Complexes in Aqueous Solution
- PMID: 20730030
- PMCID: PMC2922774
- DOI: 10.1002/ejic.201000309
Strong Circularly Polarized Luminescence from Highly Emissive Terbium Complexes in Aqueous Solution
Abstract
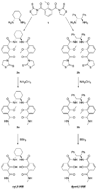
Two luminescent terbium(III) complexes have been prepared from chiral ligands containing 2-hydroxyisophthalamide (IAM) antenna chromophores and their non-polarized and circularly-polarized luminescence properties have been studied. These tetradentate ligands, which form 2:1 ligand/Tb(III) complexes, utilize diaminocyclohexane (cyLI) and diphenylethylenediamine (dpenLI) backbones, which we reasoned would impart conformational rigidity and result in Tb(III) complexes that display both large luminescence quantum yield (Φ) values and strong circularly polarized luminescence (CPL) activities. Both Tb(III) complexes are highly emissive, with Φ values of 0.32 (dpenLI-Tb) and 0.60 (cyLI-Tb). Luminescence lifetime measurements in H(2)O and D(2)O indicate that while cyLI-Tb exists as a single species in solution, dpenLI-Tb exists as two species: a monohydrate complex with one H(2)O molecule directly bound to the Tb(III) ion and a complex with no water molecules in the inner coordination sphere. Both cyLI-Tb and dpenLI-Tb display increased CPL activity compared to previously reported Tb(III) complexes made with chiral IAM ligands. The CPL measurements also provide additional confirmation of the presence of a single emissive species in solution in the case of cyLI-Tb, and multiple emissive species in the case of dpenLI-Tb.
Figures




References
-
- Brittain HG. In: Lanthanide Probes in Life, Chemical and Earth Sciences: Theory and Practice. 3rd ed. Bünzli J-CG, Choppin GR, editors. Amsterdam: Elsevier; 1989.
-
- Richardson FS, Riehl JP. Chem. Rev. 1977;77:772–792.
-
- Riehl JP, Richardson FS. Chem. Rev. 1986;86:1–16.
-
- Riehl JP, Richardson FS. Methods Enzymol. 1993;226:539–553. - PubMed
-
- Brittain HG. Appl. Spectrosc. Rev. 2007;35:175–201.

