Mechanistic studies of Sansalvamide A-amide: an allosteric modulator of Hsp90
- PMID: 20730035
- PMCID: PMC2922868
- DOI: 10.1021/ml900003t
Mechanistic studies of Sansalvamide A-amide: an allosteric modulator of Hsp90
Abstract
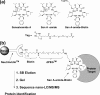
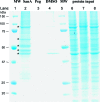
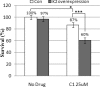
Herein we show that San A-amide, a structurally unique molecule, influences a subset of cancer-related pathways involving Hsp90. We show that San A-amide specifically binds to the N-middle domain of Hsp90 allosterically disrupts the binding of proteins thought to interact with the Hsp90 C-terminal domain, while having no effect on an N-terminal domain client protein. This unique mechanism suggests that San A-amide is a potential tool for studying C-terminal binding proteins of Hsp90 as well as a promising lead in the development of new cancer therapeutics.
Figures


References
-
- Cueto M.; Jensen P. R.; Fenical W. N-Methylsansalvamide, a cytotoxic cyclic depsipeptide from a marine fungus of the genus Fusarium. Phytochemistry 2000, 55, 223–226. - PubMed
-
- Belofsky G. N.; Jensen P. R.; Fenical W. Sansalvamide: A new cytotoxic cyclic depsipeptide produced by a marine fungus of the genus Fusarium. Tetrahedron Lett. 1999, 40, 2913–2916.
-
- Hwang Y.; Rowley D.; Rhodes D.; Gertsch J.; Fenical W.; Bushman F. Mechanism of Inhibition of a Poxvirus Topoisomerase by the Marine Natural Product Sansalvamide A. Mol. Pharmacol. 1999, 55, 1049–1053. - PubMed
-
- Otrubova K.; Lushington G. H.; Vander Velde D.; McGuire K. L.; McAlpine S. R. A Comprehensive Study of Sansalvamide A Derivatives and Their Structure-Activity Relationships against Drug-Resistant Colon Cancer Cell Lines. J. Med. Chem. 2008, 51, 530–544. - PubMed
-
- Pan P. S.; Vasko R. C.; Lapera S. A.; Johnson V. A.; Sellers R. P.; Lin C.-C.; Pan C.-M.; Davis M. R.; Ardi V. C.; McAlpine S. R. A comprehensive study of Sansalvamide A derivatives: Their structure-activity relationships and their binding mode to Hsp90. Bioorg. Med. Chem. 2009, 17, 5806–5825. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

