Using a virtual cortical module implementing a neural field model to modulate brain rhythms in Parkinson's disease
- PMID: 20730081
- PMCID: PMC2920509
- DOI: 10.3389/fnins.2010.00045
Using a virtual cortical module implementing a neural field model to modulate brain rhythms in Parkinson's disease
Abstract
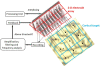
We propose a new method for selective modulation of cortical rhythms based on neural field theory, in which the activity of a cortical area is extensively monitored using a two-dimensional microelectrode array. The example of Parkinson's disease illustrates the proposed method, in which a neural field model is assumed to accurately describe experimentally recorded activity. In addition, we propose a new closed-loop stimulation signal that is both space- and time- dependent. This method is especially designed to specifically modulate a targeted brain rhythm, without interfering with other rhythms. A new class of neuroprosthetic devices is also proposed, in which the multielectrode array is seen as an artificial neural network interacting with biological tissue. Such a bio-inspired approach may provide a solution to optimize interactions between the stimulation device and the cortex aiming to attenuate or augment specific cortical rhythms. The next step will be to validate this new approach experimentally in patients with Parkinson's disease.
Keywords: Parkinson's disease; brain stimulation; dysrhythmia; neural field model; neuroprosthetic devices.
Figures





Similar articles
-
Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson's disease.J Neurosurg. 2018 Feb;128(2):605-616. doi: 10.3171/2016.11.JNS161162. Epub 2017 Apr 14. J Neurosurg. 2018. PMID: 28409730 Free PMC article.
-
High Frequency Deep Brain Stimulation and Neural Rhythms in Parkinson's Disease.Neuropsychol Rev. 2015 Dec;25(4):384-97. doi: 10.1007/s11065-015-9308-7. Epub 2015 Nov 25. Neuropsychol Rev. 2015. PMID: 26608605 Review.
-
Abnormal phasic activity in saliency network, motor areas, and basal ganglia in Parkinson's disease during rhythm perception.Hum Brain Mapp. 2019 Feb 15;40(3):916-927. doi: 10.1002/hbm.24421. Epub 2018 Oct 29. Hum Brain Mapp. 2019. PMID: 30375107 Free PMC article.
-
Unified neural field theory of brain dynamics underlying oscillations in Parkinson's disease and generalized epilepsies.J Theor Biol. 2017 Sep 7;428:132-146. doi: 10.1016/j.jtbi.2017.06.016. Epub 2017 Jun 17. J Theor Biol. 2017. PMID: 28633970
-
Treatment of Parkinson's disease by cortical stimulation.Expert Rev Neurother. 2009 Dec;9(12):1755-71. doi: 10.1586/ern.09.132. Expert Rev Neurother. 2009. PMID: 19951135 Review.
Cited by
-
Suppressing epileptic activity in a neural mass model using a closed-loop proportional-integral controller.Sci Rep. 2016 Jun 7;6:27344. doi: 10.1038/srep27344. Sci Rep. 2016. PMID: 27273563 Free PMC article.
-
Neural mass modeling of power-line magnetic fields effects on brain activity.Front Comput Neurosci. 2013 Apr 11;7:34. doi: 10.3389/fncom.2013.00034. eCollection 2013. Front Comput Neurosci. 2013. PMID: 23596412 Free PMC article.
-
Evaluating reverse speech as a control task with language-related gamma activity on electrocorticography.Neuroimage. 2012 May 1;60(4):2335-45. doi: 10.1016/j.neuroimage.2012.02.040. Epub 2012 Feb 23. Neuroimage. 2012. PMID: 22387167 Free PMC article.
-
Closed-loop stimulation of a delayed neural fields model of parkinsonian STN-GPe network: a theoretical and computational study.Front Neurosci. 2015 Jul 10;9:237. doi: 10.3389/fnins.2015.00237. eCollection 2015. Front Neurosci. 2015. PMID: 26217171 Free PMC article.
-
Explaining the heterogeneity of functional connectivity findings in multiple sclerosis: An empirically informed modeling study.Hum Brain Mapp. 2018 Jun;39(6):2541-2548. doi: 10.1002/hbm.24020. Epub 2018 Feb 21. Hum Brain Mapp. 2018. PMID: 29468785 Free PMC article.
References
-
- Atay F. M., Hutt A. (2005). Stability and bifurcations in neural fields with finite propagation speed and general connectivity. SIAM J. Appl. Math. 65, 644–66610.1137/S0036139903430884 - DOI
-
- Bressloff P. C. (2001). Traveling fronts and wave propagation failure in an inhomogeneous neural network. Physica D 155, 83–10010.1016/S0167-2789(01)00266-4 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

