Immunity against Neisseria meningitidis serogroup C in the Dutch population before and after introduction of the meningococcal c conjugate vaccine
- PMID: 20730091
- PMCID: PMC2921331
- DOI: 10.1371/journal.pone.0012144
Immunity against Neisseria meningitidis serogroup C in the Dutch population before and after introduction of the meningococcal c conjugate vaccine
Abstract
Background: In 2002 a Meningococcal serogroup C (MenC) conjugate vaccine, with tetanus toxoid as carrier protein, was introduced in the Netherlands as a single-dose at 14 months of age. A catch-up campaign was performed targeting all individuals aged 14 months to 18 years. We determined the MenC-specific immunity before and after introduction of the MenC conjugate (MenCC) vaccine.
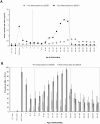
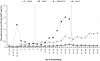
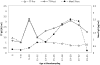
Methods and findings: Two cross-sectional population-based serum banks, collected in 1995/1996 (n = 8539) and in 2006/2007 (n = 6386), were used for this study. The main outcome measurements were the levels of MenC polysaccharide(PS)-specific IgG and serum bactericidal antibodies (SBA) after routine immunization, 4-5 years after catch-up immunization or by natural immunity. There was an increasing persistence of PS-specific IgG and SBA with age in the catch-up immunized cohorts 4-5 years after their MenCC immunization (MenC PS-specific IgG, 0.25 microg/ml (95%CI: 0.19-0.31 microg/ml) at age 6 years, gradually increasing to 2.34 microg/ml,(95%CI: 1.70-3.32 microg/ml) at age 21-22 years). A comparable pattern was found for antibodies against the carrier protein in children immunized above 9 years of age. In case of vaccination before the age of 5 years, PS-specific IgG was rapidly lost. For all age-cohorts together, SBA seroprevalence (> or =8) increased from 19.7% to 43.0% in the pre- and post-MenC introduction eras, respectively. In non-immunized adults the SBA seroprevalence was not significantly different between the pre- and post-MenC introduction periods, whereas PS-specific IgG was significantly lower in the post-MenC vaccination (GMT, age > or =25 years, 0.10 microg/ml) era compared to the pre-vaccination (GMT, age > or =25 years, 0.43 microg/ml) era.
Conclusion: MenCC vaccination administered above 5 years of age induced high IgG levels compared to natural exposure, increasing with age. In children below 14 months of age and non-immunized cohorts lower IgG levels were observed compared to the pre-vaccination era, whereas functional levels remained similar in adults. Whether the lower IgG poses individuals at increased risk for MenC disease should be carefully monitored. Large-scale introduction of a MenCC vaccine has led to improved protection in adolescents, but in infants a single-dose schedule may not provide sufficient protection on the long-term and therefore a booster-dose early in adolescence should be considered.
Conflict of interest statement
Figures
References
-
- de Greeff SC, de Melker HE, Spanjaard L, Schouls LM, van Derende A. Protection from routine vaccination at the age of 14 months with meningococcal serogroup C conjugate vaccine in the Netherlands. Pediatr Infect Dis J. 2006;25:79–80. - PubMed
-
- Gezondheidsraad/Health Council of the Netherlands. Universal vaccination against meningococcal serogroup C and pneumococcal disease. 2002. No. 2001/27E, The Hague.
-
- Neppelenbroek SE, de Vries M, de Greeff SC, Timen A. ‘da's goed gedaan? Woordverslag van de landelijke vaccinatiecampagne meningokokken C. 2002. ISBN 90-72779-38-X. 2003. GGD Nederland.
-
- Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–367. - PubMed
-
- Larrauri A, Cano R, Garcia M, Mateo S. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23:4097–4100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

