Cdc42 interaction with N-WASP and Toca-1 regulates membrane tubulation, vesicle formation and vesicle motility: implications for endocytosis
- PMID: 20730103
- PMCID: PMC2921345
- DOI: 10.1371/journal.pone.0012153
Cdc42 interaction with N-WASP and Toca-1 regulates membrane tubulation, vesicle formation and vesicle motility: implications for endocytosis
Abstract
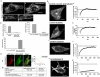
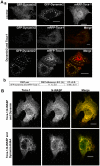
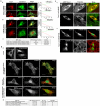
Transducer of Cdc42-dependent actin assembly (Toca-1) consists of an F-BAR domain, a Cdc42 binding site and an SH3 domain. Toca-1 interacts with N-WASP, an activator of actin nucleation that binds Cdc42. Cdc42 may play an important role in regulating Toca-1 and N-WASP functions. We report here that the cellular expression of Toca-1 and N-WASP induces membrane tubulation and the formation of motile vesicles. Marker and uptake analysis suggests that the tubules and vesicles are associated with clathrin-mediated endocytosis. Forster resonance energy transfer (FRET) and Fluorescence Lifetime Imaging Microscopy (FLIM) analysis shows that Cdc42, N-WASP and Toca-1 form a trimer complex on the membrane tubules and vesicles and that Cdc42 interaction with N-WASP is critical for complex formation. Modulation of Cdc42 interaction with Toca-1 and/or N-WASP affects membrane tubulation, vesicle formation and vesicle motility. Thus Cdc42 may influence endocytic membrane trafficking by regulating the formation and activity of the Toca-1/N-WASP complex.
Conflict of interest statement
Figures




Similar articles
-
The Toca-1-N-WASP complex links filopodial formation to endocytosis.J Biol Chem. 2009 Apr 24;284(17):11622-36. doi: 10.1074/jbc.M805940200. Epub 2009 Feb 11. J Biol Chem. 2009. PMID: 19213734 Free PMC article.
-
Regulation of neuronal morphology by Toca-1, an F-BAR/EFC protein that induces plasma membrane invagination.J Biol Chem. 2006 Sep 29;281(39):29042-53. doi: 10.1074/jbc.M604025200. Epub 2006 Aug 2. J Biol Chem. 2006. PMID: 16885158
-
EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization.EMBO J. 2008 Nov 5;27(21):2817-28. doi: 10.1038/emboj.2008.216. Epub 2008 Oct 16. EMBO J. 2008. PMID: 18923421 Free PMC article.
-
Cdc42 in actin dynamics: An ordered pathway governed by complex equilibria and directional effector handover.Small GTPases. 2017 Oct 2;8(4):237-244. doi: 10.1080/21541248.2016.1215657. Epub 2016 Aug 11. Small GTPases. 2017. PMID: 27715449 Free PMC article. Review.
-
Regulation of WASP: PIP2 Pipped by Toca-1?Cell. 2004 Jul 23;118(2):140-1. doi: 10.1016/j.cell.2004.07.005. Cell. 2004. PMID: 15260983 Review.
Cited by
-
Roles of rho GTPases in intracellular transport and cellular transformation.Int J Mol Sci. 2013 Mar 28;14(4):7089-108. doi: 10.3390/ijms14047089. Int J Mol Sci. 2013. PMID: 23538840 Free PMC article. Review.
-
Control of Rho GTPase function by BAR-domains.Small GTPases. 2012 Jan-Mar;3(1):45-52. doi: 10.4161/sgtp.18960. Small GTPases. 2012. PMID: 22714417 Free PMC article.
-
Myelin is dependent on the Charcot-Marie-Tooth Type 4H disease culprit protein FRABIN/FGD4 in Schwann cells.Brain. 2012 Dec;135(Pt 12):3567-83. doi: 10.1093/brain/aws275. Epub 2012 Nov 20. Brain. 2012. PMID: 23171661 Free PMC article.
-
A conserved protein tyrosine phosphatase, PTPN-22, functions in diverse developmental processes in C. elegans.bioRxiv [Preprint]. 2024 Mar 15:2024.03.12.584557. doi: 10.1101/2024.03.12.584557. bioRxiv. 2024. Update in: PLoS Genet. 2024 Aug 22;20(8):e1011219. doi: 10.1371/journal.pgen.1011219. PMID: 38559252 Free PMC article. Updated. Preprint.
-
Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling.J Cell Biol. 2011 Mar 21;192(6):907-17. doi: 10.1083/jcb.201009141. J Cell Biol. 2011. PMID: 21422226 Free PMC article. Review.
References
-
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. - PubMed
-
- Ellis S, Mellor H. Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol. 2000;10:85–88. - PubMed
-
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. - PubMed
-
- Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

