Tumorigenic WAP-T mouse mammary carcinoma cells: a model for a self-reproducing homeostatic cancer cell system
- PMID: 20730114
- PMCID: PMC2920333
- DOI: 10.1371/journal.pone.0012103
Tumorigenic WAP-T mouse mammary carcinoma cells: a model for a self-reproducing homeostatic cancer cell system
Abstract
Background: In analogy to normal stem cell differentiation, the current cancer stem cell (CSC) model presumes a hierarchical organization and an irreversible differentiation in tumor tissue. Accordingly, CSCs should comprise only a small subset of the tumor cells, which feeds tumor growth. However, some recent findings raised doubts on the general applicability of the CSC model and asked for its refinement.
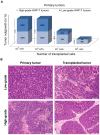
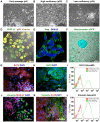
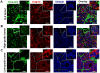
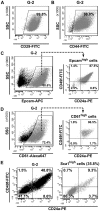
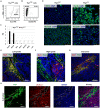
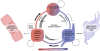
Methodology/principal findings: In this study we analyzed the CSC properties of mammary carcinoma cells derived from transgenic (WAP-T) mice. We established a highly tumorigenic WAP-T cell line (G-2 cells) that displays stem-like traits. G-2 cells, as well as their clonal derivates, are closely related to primary tumors regarding histology and gene expression profiles, and reflect heterogeneity regarding their differentiation states. G-2 cultures comprise cell populations in distinct differentiation states identified by co-expression of cytoskeletal proteins (cytokeratins and vimentin), a combination of cell surface markers and a set of transcription factors. Cellular subsets sorted according to expression of CD24a, CD49f, CD61, Epcam, Sca1, and Thy1 cell surface proteins, or metabolic markers (e.g. ALDH activity) are competent to reconstitute the initial cellular composition. Repopulation efficiency greatly varies between individual subsets and is influenced by interactions with the respective complementary G-2 cellular subset. The balance between differentiation states is regulated in part by the transcription factor Sox10, as depletion of Sox10 led to up-regulation of Twist2 and increased the proportion of Thy1-expressing cells representing cells in a self-renewable, reversible, quasi-mesenchymal differentiation state.
Conclusions/significance: G-2 cells constitute a self-reproducing cancer cell system, maintained by bi- and unidirectional conversion of complementary cellular subsets. Our work contributes to the current controversial discussion on the existence and nature of CSC and provides a basis for the incorporation of alternative hypotheses into the CSC model.
Conflict of interest statement
Figures



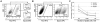




Similar articles
-
Epithelial-mesenchymal plasticity is a decisive feature for the metastatic outgrowth of disseminated WAP-T mouse mammary carcinoma cells.BMC Cancer. 2015 Mar 26;15:178. doi: 10.1186/s12885-015-1165-5. BMC Cancer. 2015. PMID: 25886487 Free PMC article.
-
Targeted Pten deletion plus p53-R270H mutation in mouse mammary epithelium induces aggressive claudin-low and basal-like breast cancer.Breast Cancer Res. 2016 Jan 19;18(1):9. doi: 10.1186/s13058-015-0668-y. Breast Cancer Res. 2016. PMID: 26781438 Free PMC article.
-
Human non-small cell lung cancer expresses putative cancer stem cell markers and exhibits the transcriptomic profile of multipotent cells.BMC Cancer. 2015 Feb 25;15:84. doi: 10.1186/s12885-015-1086-3. BMC Cancer. 2015. PMID: 25881239 Free PMC article.
-
Understanding of tumourigenesis in canine mammary tumours based on cancer stem cell research.Vet J. 2020 Nov;265:105560. doi: 10.1016/j.tvjl.2020.105560. Epub 2020 Oct 4. Vet J. 2020. PMID: 33129557 Review.
-
Cancer cells in epithelial-to-mesenchymal transition and tumor-propagating-cancer stem cells: distinct, overlapping or same populations.Oncogene. 2011 Nov 17;30(46):4609-21. doi: 10.1038/onc.2011.184. Epub 2011 Jun 6. Oncogene. 2011. PMID: 21643013 Review.
Cited by
-
HDAC8 suppresses the epithelial phenotype and promotes EMT in chemotherapy-treated basal-like breast cancer.Clin Epigenetics. 2022 Jan 11;14(1):7. doi: 10.1186/s13148-022-01228-4. Clin Epigenetics. 2022. PMID: 35016723 Free PMC article.
-
T-cell epitope strength in WAP-T mouse mammary carcinomas is an important determinant in PD1/PD-L1 immune checkpoint blockade therapy.Oncotarget. 2016 Oct 4;7(40):64543-64559. doi: 10.18632/oncotarget.11620. Oncotarget. 2016. PMID: 27579535 Free PMC article.
-
Epithelial-mesenchymal plasticity is a decisive feature for the metastatic outgrowth of disseminated WAP-T mouse mammary carcinoma cells.BMC Cancer. 2015 Mar 26;15:178. doi: 10.1186/s12885-015-1165-5. BMC Cancer. 2015. PMID: 25886487 Free PMC article.
-
Infiltration of Immune Competent Cells into Primary Tumors and Their Surrounding Connective Tissues in Xenograft and Syngeneic Mouse Models.Int J Mol Sci. 2021 Apr 19;22(8):4213. doi: 10.3390/ijms22084213. Int J Mol Sci. 2021. PMID: 33921688 Free PMC article.
-
Immunotherapy of WAP-TNP mice with early stage mammary gland tumors.Oncotarget. 2017 Jun 29;8(40):67790-67804. doi: 10.18632/oncotarget.18850. eCollection 2017 Sep 15. Oncotarget. 2017. PMID: 28978072 Free PMC article.
References
-
- Rubin H. Ordered heterogeneity and its decline in cancer and aging. Adv Cancer Res. 2007;98:117–147. - PubMed
-
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. - PubMed
-
- Ward RJ, Dirks PB. Cancer Stem Cells: At the Headwaters of Tumor Development. Annu Rev Pathol. 2007;2:175–189. - PubMed
-
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. - PubMed
-
- Nowell PC. Mechanisms of tumor progression. Cancer Res. 1986;46:2203–2207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

