Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit
- PMID: 20730946
- PMCID: PMC2948245
- DOI: 10.1002/bies.201000042
Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit
Abstract
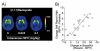
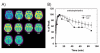
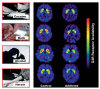
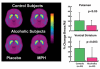
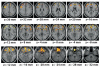
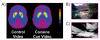
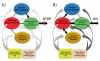
Based on brain imaging findings, we present a model according to which addiction emerges as an imbalance in the information processing and integration among various brain circuits and functions. The dysfunctions reflect (a) decreased sensitivity of reward circuits, (b) enhanced sensitivity of memory circuits to conditioned expectations to drugs and drug cues, stress reactivity, and (c) negative mood, and a weakened control circuit. Although initial experimentation with a drug of abuse is largely a voluntary behavior, continued drug use can eventually impair neuronal circuits in the brain that are involved in free will, turning drug use into an automatic compulsive behavior. The ability of addictive drugs to co-opt neurotransmitter signals between neurons (including dopamine, glutamate, and GABA) modifies the function of different neuronal circuits, which begin to falter at different stages of an addiction trajectory. Upon exposure to the drug, drug cues or stress this results in unrestrained hyperactivation of the motivation/drive circuit that results in the compulsive drug intake that characterizes addiction.
Figures

Similar articles
-
Addiction and brain reward and antireward pathways.Adv Psychosom Med. 2011;30:22-60. doi: 10.1159/000324065. Epub 2011 Apr 19. Adv Psychosom Med. 2011. PMID: 21508625 Free PMC article. Review.
-
Dopamine homeostasis: brain functional connectivity in reward deficiency syndrome.Front Biosci (Landmark Ed). 2017 Jan 1;22(4):669-691. doi: 10.2741/4509. Front Biosci (Landmark Ed). 2017. PMID: 27814639 Review.
-
Dual roles of dopamine in food and drug seeking: the drive-reward paradox.Biol Psychiatry. 2013 May 1;73(9):819-26. doi: 10.1016/j.biopsych.2012.09.001. Epub 2012 Oct 5. Biol Psychiatry. 2013. PMID: 23044182 Free PMC article. Review.
-
Addiction and stress: Exploring the reward pathways in brain affected by different drugs.Prog Brain Res. 2025;291:381-404. doi: 10.1016/bs.pbr.2025.01.012. Epub 2025 Jan 31. Prog Brain Res. 2025. PMID: 40222788 Review.
-
Transitionality in addiction: A "temporal continuum" hypotheses involving the aberrant motivation, the hedonic dysregulation, and the aberrant learning.Med Hypotheses. 2016 Aug;93:62-70. doi: 10.1016/j.mehy.2016.05.015. Epub 2016 May 17. Med Hypotheses. 2016. PMID: 27372858
Cited by
-
Differential behavioral and molecular alterations upon protracted abstinence from cocaine versus morphine, nicotine, THC and alcohol.Addict Biol. 2017 Sep;22(5):1205-1217. doi: 10.1111/adb.12405. Epub 2016 Apr 28. Addict Biol. 2017. PMID: 27126842 Free PMC article.
-
Altered default network resting-state functional connectivity in adolescents with Internet gaming addiction.PLoS One. 2013;8(3):e59902. doi: 10.1371/journal.pone.0059902. Epub 2013 Mar 26. PLoS One. 2013. PMID: 23555827 Free PMC article.
-
Addiction Vulnerability and Binge Eating in Women: Exploring Reward Sensitivity, Affect Regulation, Impulsivity & Weight/Shape Concerns.Pers Individ Dif. 2016 Oct;100:16-22. doi: 10.1016/j.paid.2016.03.084. Epub 2016 Apr 13. Pers Individ Dif. 2016. PMID: 27773957 Free PMC article.
-
Loss in connectivity among regions of the brain reward system in alcohol dependence.Hum Brain Mapp. 2013 Dec;34(12):3129-42. doi: 10.1002/hbm.22132. Epub 2012 Jul 19. Hum Brain Mapp. 2013. PMID: 22815206 Free PMC article.
-
Protracted abstinence in males with an opioid use disorder: partial recovery of nucleus accumbens function.Transl Psychiatry. 2022 Feb 26;12(1):81. doi: 10.1038/s41398-022-01813-4. Transl Psychiatry. 2022. PMID: 35217657 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

