Kinetic analysis reveals differences in the binding mechanism of calmodulin and calmodulin-like protein to the IQ motifs of myosin-10
- PMID: 20731332
- PMCID: PMC3818122
- DOI: 10.1021/bi100644q
Kinetic analysis reveals differences in the binding mechanism of calmodulin and calmodulin-like protein to the IQ motifs of myosin-10
Abstract
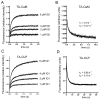
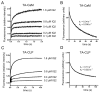
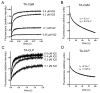
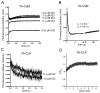
Myo10 is an unconventional myosin with important functions in filopodial motility, cell migration, and cell adhesion. The neck region of Myo10 contains three IQ motifs that bind calmodulin (CaM) or the tissue-restricted calmodulin-like protein (CLP) as light chains. However, little is known about the mechanism of light chain binding to the IQ motifs in Myo10. Binding of CaM and CLP to each IQ motif was assessed by nondenaturing gel electrophoresis and by stopped-flow experiments using fluorescence-labeled CaM and CLP. Although the binding kinetics are different in each case, there are similarities in the mechanism of binding of CaM and CLP to IQ1 and IQ2: for both IQ motifs Ca(2+) increased the binding affinity, mainly by increasing the rate of the forward steps. The general kinetic mechanism comprises a two-step process, which in some cases may involve the binding of a second IQ motif with lower affinity. For IQ3, however, the kinetics of CaM binding is very different from that of CLP. In both cases, binding in the absence of Ca(2+) is poor, and addition of Ca(2+) decreases the K(d) to below 10 nM. However, while the CaM binding kinetics are complex and best fitted by a multistep model, binding of CLP is fitted by a relatively simple two-step model. The results show that, in keeping with growing structural evidence, complexes between CaM or CaM-like myosin light chains and IQ motifs are highly diverse and depend on the specific sequence of the particular IQ motif as well as the light chain.
Figures





Similar articles
-
Interaction with the IQ3 motif of myosin-10 is required for calmodulin-like protein-dependent filopodial extension.FEBS Lett. 2008 Jul 9;582(16):2377-81. doi: 10.1016/j.febslet.2008.05.051. Epub 2008 Jun 18. FEBS Lett. 2008. PMID: 18570893
-
A model of Ca(2+)-free calmodulin binding to unconventional myosins reveals how calmodulin acts as a regulatory switch.Structure. 1996 Dec 15;4(12):1475-90. doi: 10.1016/s0969-2126(96)00154-2. Structure. 1996. PMID: 8994973
-
Calmodulin bound to the first IQ motif is responsible for calcium-dependent regulation of myosin 5a.J Biol Chem. 2012 May 11;287(20):16530-40. doi: 10.1074/jbc.M112.343079. Epub 2012 Mar 21. J Biol Chem. 2012. PMID: 22437832 Free PMC article.
-
Calmodulin signaling via the IQ motif.FEBS Lett. 2002 Feb 20;513(1):107-13. doi: 10.1016/s0014-5793(01)03239-2. FEBS Lett. 2002. PMID: 11911888 Review.
-
Sequence motifs for calmodulin recognition.FASEB J. 1997 Apr;11(5):331-40. doi: 10.1096/fasebj.11.5.9141499. FASEB J. 1997. PMID: 9141499 Review.
Cited by
-
Emanuel Strehler's work on calcium pumps and calcium signaling.World J Biol Chem. 2011 Apr 26;2(4):67-72. doi: 10.4331/wjbc.v2.i4.67. World J Biol Chem. 2011. PMID: 21537475 Free PMC article.
-
Endothelial IQGAP1 regulates leukocyte transmigration by directing the LBRC to the site of diapedesis.J Exp Med. 2019 Nov 4;216(11):2582-2601. doi: 10.1084/jem.20190008. Epub 2019 Aug 8. J Exp Med. 2019. PMID: 31395618 Free PMC article.
-
Myosin-X: a MyTH-FERM myosin at the tips of filopodia.J Cell Sci. 2011 Nov 15;124(Pt 22):3733-41. doi: 10.1242/jcs.023549. J Cell Sci. 2011. PMID: 22124140 Free PMC article. Review.
-
Myosin light chains: Teaching old dogs new tricks.Bioarchitecture. 2014;4(6):169-88. doi: 10.1080/19490992.2015.1054092. Bioarchitecture. 2014. PMID: 26155737 Free PMC article. Review.
References
-
- Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci. 2000;113:3439–3451. - PubMed
-
- Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nature Cell Biol. 2002;4:246–250. - PubMed
-
- Yonezawa S, Yoshizaki N, Sano M, Hanai A, Masaki S, Takizawa T, Kageyama T, Moriyama A. Possible involvement of myosin-X in intercellular adhesion: importance of serial pleckstrin homology regions for intracellular localization. Develop Growth Differ. 2003;45:175–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

