Solution structure of the 128 kDa enzyme I dimer from Escherichia coli and its 146 kDa complex with HPr using residual dipolar couplings and small- and wide-angle X-ray scattering
- PMID: 20731394
- PMCID: PMC2955445
- DOI: 10.1021/ja105485b
Solution structure of the 128 kDa enzyme I dimer from Escherichia coli and its 146 kDa complex with HPr using residual dipolar couplings and small- and wide-angle X-ray scattering
Abstract
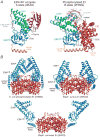
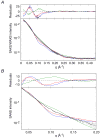
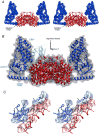
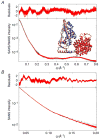
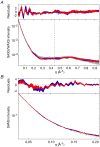
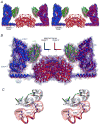
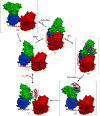
The solution structures of free Enzyme I (EI, ∼128 kDa, 575 × 2 residues), the first enzyme in the bacterial phosphotransferase system, and its complex with HPr (∼146 kDa) have been solved using novel methodology that makes use of prior structural knowledge (namely, the structures of the dimeric EIC domain and the isolated EIN domain both free and complexed to HPr), combined with residual dipolar coupling (RDC), small- (SAXS) and wide- (WAXS) angle X-ray scattering and small-angle neutron scattering (SANS) data. The calculational strategy employs conjoined rigid body/torsion/Cartesian simulated annealing, and incorporates improvements in calculating and refining against SAXS/WAXS data that take into account complex molecular shapes in the description of the solvent layer resulting in a better representation of the SAXS/WAXS data. The RDC data orient the symmetrically related EIN domains relative to the C(2) symmetry axis of the EIC dimer, while translational, shape, and size information is provided by SAXS/WAXS. The resulting structures are independently validated by SANS. Comparison of the structures of the free EI and the EI-HPr complex with that of the crystal structure of a trapped phosphorylated EI intermediate reveals large (∼70-90°) hinge body rotations of the two subdomains comprising the EIN domain, as well as of the EIN domain relative to the dimeric EIC domain. These large-scale interdomain motions shed light on the structural transitions that accompany the catalytic cycle of EI.
Figures






References
-
- Meadow ND, Fox DK, Roseman S. Annu Rev Biochem. 1990;59:497–542. - PubMed
-
- Robillard GT, Broos J. Biochim Biophys Acta. 1999;1422:73–104. - PubMed
-
- Siebold C, Flukiger K, Beutler R, Erni B. FEBS Lett. 2001;504:104–111. - PubMed
-
- Weigel N, Waygood EB, Kukuruzinska MA, Nakazawa A, Roseman S. J Biol Chem. 1982;257:14461–14469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

