Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine
- PMID: 20731720
- PMCID: PMC3025045
- DOI: 10.1111/j.1601-183X.2010.00641.x
Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine
Abstract
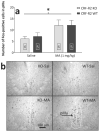
Sensitivity to the euphoric and locomotor-activating effects of drugs of abuse may contribute to risk for excessive use and addiction. Repeated administration of psychostimulants such as methamphetamine (MA) can result in neuroadaptive consequences that manifest behaviorally as a progressive escalation of locomotor activation, termed psychomotor sensitization. The present studies addressed the involvement of specific components of the corticotropin-releasing factor (CRF) system in locomotor activation and psychomotor sensitization induced by MA (1, 2 mg/kg) by utilizing pharmacological approaches, as well as a series of genetic knockout (KO) mice, each deficient for a single component of the CRF system: CRF-R1, CRF-R2, CRF, or the CRF-related peptide Urocortin 1 (Ucn1). CRF-R1 KO mice did not differ from wild-type mice in sensitization to MA, and pharmacological blockade of CRF-R1 with CP-154,526 (15, 30 mg/kg) in DBA/2J mice did not selectively attenuate either the acquisition or expression of MA-induced sensitization. Deletion of either of the endogenous ligands of CRF-R1 (CRF, Ucn1) either enhanced or had no effect on MA-induced sensitization, providing further evidence against a role for CRF-R1 signaling. Interestingly, deletion of CRF-R2 attenuated MA-induced locomotor activation, elucidating a novel contribution of the CRF system to MA sensitivity, and suggesting the participation of the endogenous urocortin peptides Ucn2 and Ucn3. Immunohistochemistry for Fos was used to visualize neural activation underlying CRF-R2-dependent sensitivity to MA, identifying the basolateral and central nuclei of the amygdala as neural substrates involved in this response. Our results support further examination of CRF-R2 involvement in neural processes associated with MA addiction.
© 2010 The Authors. Genes, Brain and Behavior © 2010 Blackwell Publishing Ltd and International Behavioural and Neural Genetics Society.
Figures
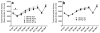

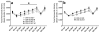
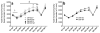
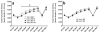



Similar articles
-
Dissociation of corticotropin-releasing factor receptor subtype involvement in sensitivity to locomotor effects of methamphetamine and cocaine.Psychopharmacology (Berl). 2012 Feb;219(4):1055-63. doi: 10.1007/s00213-011-2433-y. Epub 2011 Aug 11. Psychopharmacology (Berl). 2012. PMID: 21833501 Free PMC article.
-
Role of corticotropin-releasing factor and corticosterone in behavioral sensitization to ethanol.J Pharmacol Exp Ther. 2012 May;341(2):455-63. doi: 10.1124/jpet.111.190595. Epub 2012 Feb 14. J Pharmacol Exp Ther. 2012. PMID: 22333290 Free PMC article.
-
Corticotropin-releasing factor acting on corticotropin-releasing factor receptor type 1 is critical for binge alcohol drinking in mice.Alcohol Clin Exp Res. 2012 Feb;36(2):369-76. doi: 10.1111/j.1530-0277.2011.01610.x. Epub 2011 Sep 6. Alcohol Clin Exp Res. 2012. PMID: 21895713 Free PMC article.
-
Contribution of Urocortin to the Development of Excessive Drinking.Int Rev Neurobiol. 2017;136:275-291. doi: 10.1016/bs.irn.2017.06.007. Epub 2017 Aug 2. Int Rev Neurobiol. 2017. PMID: 29056154 Review.
-
The suppression effects of feeding and mechanisms in CRF system of animals.Gene. 2020 Apr 5;733:144363. doi: 10.1016/j.gene.2020.144363. Epub 2020 Jan 11. Gene. 2020. PMID: 31935510 Review.
Cited by
-
Effects of sodium butyrate on methamphetamine-sensitized locomotor activity.Behav Brain Res. 2013 Feb 15;239:139-47. doi: 10.1016/j.bbr.2012.10.046. Epub 2012 Nov 5. Behav Brain Res. 2013. PMID: 23137698 Free PMC article.
-
Urocortin-1 within the centrally-projecting Edinger-Westphal nucleus is critical for ethanol preference.PLoS One. 2011;6(10):e26997. doi: 10.1371/journal.pone.0026997. Epub 2011 Oct 28. PLoS One. 2011. PMID: 22046429 Free PMC article.
-
Neural circuit mechanisms of the cholecystokinin (CCK) neuropeptide system in addiction.Addict Neurosci. 2022 Sep;3:100024. doi: 10.1016/j.addicn.2022.100024. Epub 2022 Jun 17. Addict Neurosci. 2022. PMID: 35983578 Free PMC article.
-
Sex differences in activation of the hypothalamic-pituitary-adrenal axis by methamphetamine.J Neurochem. 2014 May;129(3):495-508. doi: 10.1111/jnc.12651. Epub 2014 Feb 10. J Neurochem. 2014. PMID: 24400874 Free PMC article.
-
Control of chronic excessive alcohol drinking by genetic manipulation of the Edinger-Westphal nucleus urocortin-1 neuropeptide system.Transl Psychiatry. 2017 Jan 31;7(1):e1021. doi: 10.1038/tp.2016.293. Transl Psychiatry. 2017. PMID: 28140406 Free PMC article.
References
-
- Atkins AL, Helms ML, O'Toole LA, Belknap JK. Stereotypic behaviors in mice selectively bred for high and low methamphetamine-induced stereotypic chewing. Psychopharmacology (Berl) 2001;157:96–104. - PubMed
-
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. - PubMed
-
- Borowsky B, Kuhn CM. Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J Pharmacol Exp Ther. 1991;256:204–210. - PubMed
-
- Cador M, Cole BJ, Koob GF, Stinus L, Le Moal M. Central administration of corticotropin releasing factor induces long-term sensitization to D-amphetamine. Brain Res. 1993a;606:181–186. - PubMed
-
- Cador M, Dulluc J, Mormede P. Modulation of the locomotor response to amphetamine by corticosterone. Neuroscience. 1993b;56:981–988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

