Bex1 is involved in the regeneration of axons after injury
- PMID: 20731761
- PMCID: PMC3382973
- DOI: 10.1111/j.1471-4159.2010.06960.x
Bex1 is involved in the regeneration of axons after injury
Abstract
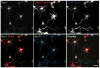
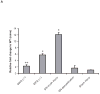
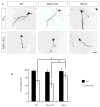
Successful axonal regeneration is a complex process determined by both axonal environment and endogenous neural capability of the regenerating axons in the central and the peripheral nervous systems. Numerous external inhibitory factors inhibit axonal regeneration after injury. In response, neurons express various regeneration-associated genes to overcome this inhibition and increase the intrinsic growth capacity. In the present study, we show that the brain-expressed X-linked (Bex1) protein was over-expressed as a result of peripheral axonal damage. Bex1 antagonized the axon outgrowth inhibitory effect of myelin-associated glycoprotein. The involvement of Bex1 in axon regeneration was further confirmed in vivo. We have demonstrated that Bex1 knock-out mice showed lower capability for regeneration after peripheral nerve injury than wild-type animals. Wild-type mice could recover from sciatic nerve injury much faster than Bex1 knock-out mice. Our findings suggest that Bex1 could be considered as regeneration-associated gene.
© 2010 The Authors. Journal of Neurochemistry © 2010 International Society for Neurochemistry.
Figures



Similar articles
-
Analysis of axonal regeneration in the central and peripheral nervous systems of the NG2-deficient mouse.BMC Neurosci. 2007 Sep 27;8:80. doi: 10.1186/1471-2202-8-80. BMC Neurosci. 2007. PMID: 17900358 Free PMC article.
-
Selective inhibition of early axonal regeneration by myelin-associated glycoprotein.Exp Neurol. 1998 Apr;150(2):254-62. doi: 10.1006/exnr.1997.6775. Exp Neurol. 1998. PMID: 9527895
-
Soluble myelin-associated glycoprotein released from damaged white matter inhibits axonal regeneration.Mol Cell Neurosci. 2001 Sep;18(3):259-69. doi: 10.1006/mcne.2001.1020. Mol Cell Neurosci. 2001. PMID: 11591127
-
Applications of Proteomics to Nerve Regeneration Research.In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review.
-
Myelin-associated glycoprotein: a role in myelination and in the inhibition of axonal regeneration?Curr Opin Neurobiol. 1995 Oct;5(5):588-95. doi: 10.1016/0959-4388(95)80063-8. Curr Opin Neurobiol. 1995. PMID: 8580710 Review.
Cited by
-
The brain expressed x-linked gene 1 (Bex1) regulates myoblast fusion.Dev Biol. 2016 Jan 1;409(1):16-25. doi: 10.1016/j.ydbio.2015.11.007. Epub 2015 Nov 14. Dev Biol. 2016. PMID: 26586200 Free PMC article.
-
Gene targeting study reveals unexpected expression of brain-expressed X-linked 2 in endocrine and tissue stem/progenitor cells in mice.J Biol Chem. 2014 Oct 24;289(43):29892-911. doi: 10.1074/jbc.M114.580084. Epub 2014 Aug 20. J Biol Chem. 2014. PMID: 25143383 Free PMC article.
-
G proteins, p60TRP, and neurodegenerative diseases.Mol Neurobiol. 2013 Jun;47(3):1103-11. doi: 10.1007/s12035-013-8410-1. Epub 2013 Jan 24. Mol Neurobiol. 2013. PMID: 23345134 Review.
-
Single-cell and genetic multi-omics analysis combined with experiments confirmed the signature and potential targets of cuproptosis in hepatocellular carcinoma.Front Cell Dev Biol. 2023 Sep 8;11:1240390. doi: 10.3389/fcell.2023.1240390. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37745297 Free PMC article.
-
Analysis of Human Papillomavirus-Associated Cervical Cancer Differentially Expressed Genes and Identification of Prognostic Factors using Integrated Bioinformatics Approaches.Adv Biomed Res. 2024 Sep 23;13:78. doi: 10.4103/abr.abr_338_23. eCollection 2024. Adv Biomed Res. 2024. PMID: 39512411 Free PMC article.
References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat Genet. 1995;11:274–280. - PubMed
-
- Alvarez E, Zhou W, Witta SE, Freed CR. Characterization of the Bex gene family in humans, mice, and rats. Gene. 2005;357:18–28. - PubMed
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. - PubMed
-
- Azzouz M, Poindron P, Guettier S, Leclerc N, Andres C, Warter J, Borg J, Arzneimittel S. Prevention of mutant SOD1 motoneuron degeneration by copper chelators in vitro. Dev Neurobiol. 2000;42:49–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

