Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: an in vitro study
- PMID: 20731873
- PMCID: PMC2936347
- DOI: 10.1186/1471-2474-11-188
Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: an in vitro study
Abstract
Background: Although pulsed electromagnetic field (PEMF) stimulation may be clinically beneficial during fracture healing and for a wide range of bone disorders, there is still debate on its working mechanism. Mesenchymal stem cells are likely mediators facilitating the observed clinical effects of PEMF. Here, we performed in vitro experiments to investigate the effect of PEMF stimulation on human bone marrow-derived stromal cell (BMSC) metabolism and, specifically, whether PEMF can stimulate their osteogenic differentiation.
Methods: BMSCs derived from four different donors were cultured in osteogenic medium, with the PEMF treated group being continuously exposed to a 15 Hz, 1 Gauss EM field, consisting of 5-millisecond bursts with 5-microsecond pulses. On culture day 1, 5, 9, and 14, cells were collected for biochemical analysis (DNA amount, alkaline phosphatase activity, calcium deposition), expression of various osteoblast-relevant genes and activation of extracellular signal-regulated kinase (ERK) signaling. Differences between treated and control groups were analyzed using the Wilcoxon signed rank test, and considered significant when p < 0.05.
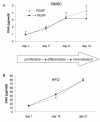
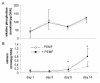
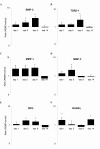
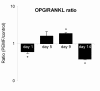
Results: Biochemical analysis revealed significant, differentiation stage-dependent, PEMF-induced differences: PEMF increased mineralization at day 9 and 14, without altering alkaline phosphatase activity. Cell proliferation, as measured by DNA amounts, was not affected by PEMF until day 14. Here, DNA content stagnated in PEMF treated group, resulting in less DNA compared to control.Quantitative RT-PCR revealed that during early culture, up to day 9, PEMF treatment increased mRNA levels of bone morphogenetic protein 2, transforming growth factor-beta 1, osteoprotegerin, matrix metalloproteinase-1 and -3, osteocalcin, and bone sialoprotein. In contrast, receptor activator of NF-κB ligand expression was primarily stimulated on day 14. ERK1/2 phosphorylation was not affected by PEMF stimulation.
Conclusions: PEMF exposure of differentiating human BMSCs enhanced mineralization and seemed to induce differentiation at the expense of proliferation. The osteogenic stimulus of PEMF was confirmed by the up-regulation of several osteogenic marker genes in the PEMF treated group, which preceded the deposition of mineral itself. These findings indicate that PEMF can directly stimulate osteoprogenitor cells towards osteogenic differentiation. This supports the theory that PEMF treatment may recruit these cells to facilitate an osteogenic response in vivo.
Figures


Similar articles
-
Differentiation of osteoprogenitor cells is induced by high-frequency pulsed electromagnetic fields.J Craniofac Surg. 2012 Mar;23(2):586-93. doi: 10.1097/SCS.0b013e31824cd6de. J Craniofac Surg. 2012. PMID: 22446422
-
Pulsed electromagnetic fields stimulate osteogenic differentiation in human bone marrow and adipose tissue derived mesenchymal stem cells.Bioelectromagnetics. 2014 Sep;35(6):426-36. doi: 10.1002/bem.21862. Epub 2014 Aug 6. Bioelectromagnetics. 2014. PMID: 25099126
-
The Effects of a Pulsed Electromagnetic Field on the Proliferation and Osteogenic Differentiation of Human Adipose-Derived Stem Cells.Med Sci Monit. 2018 May 18;24:3274-3282. doi: 10.12659/MSM.907815. Med Sci Monit. 2018. PMID: 29775452 Free PMC article.
-
Augmentation of Deficient Bone Healing by Pulsed Electromagnetic Fields-From Mechanisms to Clinical Outcomes.Bioengineering (Basel). 2024 Dec 3;11(12):1223. doi: 10.3390/bioengineering11121223. Bioengineering (Basel). 2024. PMID: 39768041 Free PMC article. Review.
-
Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response.J Am Acad Orthop Surg Glob Res Rev. 2020 May;4(5):e1900155. doi: 10.5435/JAAOSGlobal-D-19-00155. J Am Acad Orthop Surg Glob Res Rev. 2020. PMID: 33970582 Free PMC article. Review.
Cited by
-
Systemic treatment with pulsed electromagnetic fields do not affect bone microarchitecture in osteoporotic rats.Int Orthop. 2012 Jul;36(7):1501-6. doi: 10.1007/s00264-011-1471-8. Epub 2012 Jan 17. Int Orthop. 2012. PMID: 22249842 Free PMC article.
-
Physical Treatments and Therapies for Androgenetic Alopecia.J Clin Med. 2024 Aug 2;13(15):4534. doi: 10.3390/jcm13154534. J Clin Med. 2024. PMID: 39124800 Free PMC article. Review.
-
Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-caprolactone)/β-tricalcium Phosphate Composite.Int J Mol Sci. 2021 Oct 18;22(20):11216. doi: 10.3390/ijms222011216. Int J Mol Sci. 2021. PMID: 34681873 Free PMC article.
-
Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration.Stem Cell Res Ther. 2020 Feb 3;11(1):46. doi: 10.1186/s13287-020-1566-5. Stem Cell Res Ther. 2020. PMID: 32014064 Free PMC article.
-
Synergistic effect of a LPEMF and SPIONs on BMMSC proliferation, directional migration, and osteoblastogenesis.Am J Transl Res. 2018 May 15;10(5):1431-1443. eCollection 2018. Am J Transl Res. 2018. PMID: 29887957 Free PMC article.
References
-
- Heckman JD, Ingram AJ, Loyd RD, Luck JV, Jr, Mayer PW. Nonunion treatment with pulsed electromagnetic fields. Clin Orthop. 1981. pp. 58–66. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

