Epidermal growth factor targeting of bacteriophage to the choroid plexus for gene delivery to the central nervous system via cerebrospinal fluid
- PMID: 20732308
- PMCID: PMC2955767
- DOI: 10.1016/j.brainres.2010.08.044
Epidermal growth factor targeting of bacteriophage to the choroid plexus for gene delivery to the central nervous system via cerebrospinal fluid
Abstract
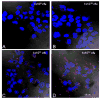
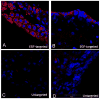
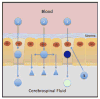
Because the choroid plexus normally controls the production and composition of cerebrospinal fluid and, as such, its many functions of the central nervous system, we investigated whether ligand-mediated targeting could deliver genes to its secretory epithelium. We show here that when bacteriophages are targeted with epidermal growth factor, they acquire the ability to enter choroid epithelial cells grown in vitro as cell cultures, ex vivo as tissue explants or in vivo by intracerebroventricular injection. The binding and internalization of these particles activate EGF receptors on targeted cells, and the dose- and time-dependent internalization of particles is inhibited by the presence of excess ligand. When the phage genome is further reengineered to contain like green fluorescent protein or firefly luciferase under control of the cytomegalovirus promoter, gene expression is detectable in the choroid plexus and ependymal epithelium by immunohistochemistry or by noninvasive imaging, respectively. Taken together, these data support the hypothesis that reengineered ligand-mediated gene delivery should be considered a viable strategy to increase the specificity of gene delivery to the central nervous system and bypass the blood-brain barrier so as to exploit the biological effectiveness of the choroid plexus as a portal of entry into the brain.
Copyright © 2010 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Targeting choroid plexus epithelia and ventricular ependyma for drug delivery to the central nervous system.BMC Neurosci. 2011 Jan 7;12:4. doi: 10.1186/1471-2202-12-4. BMC Neurosci. 2011. PMID: 21214926 Free PMC article.
-
Cardiotrophin-1 in choroid plexus and the cerebrospinal fluid circulatory system.Neuroscience. 2004;127(1):43-52. doi: 10.1016/j.neuroscience.2004.03.065. Neuroscience. 2004. PMID: 15219667
-
Transport across the choroid plexus epithelium.Am J Physiol Cell Physiol. 2017 Jun 1;312(6):C673-C686. doi: 10.1152/ajpcell.00041.2017. Epub 2017 Mar 22. Am J Physiol Cell Physiol. 2017. PMID: 28330845 Review.
-
Pathogenic implications of cerebrospinal fluid barrier pathology in neuromyelitis optica.Acta Neuropathol. 2017 Apr;133(4):597-612. doi: 10.1007/s00401-017-1682-1. Epub 2017 Feb 9. Acta Neuropathol. 2017. PMID: 28184993 Free PMC article.
-
A Hidden Epithelial Barrier in the Brain with a Central Role in Regulating Brain Homeostasis. Implications for Aging.Ann Am Thorac Soc. 2016 Dec;13 Suppl 5:S407-S410. doi: 10.1513/AnnalsATS.201609-676AW. Ann Am Thorac Soc. 2016. PMID: 28005425 Review.
Cited by
-
Targeting choroid plexus epithelia and ventricular ependyma for drug delivery to the central nervous system.BMC Neurosci. 2011 Jan 7;12:4. doi: 10.1186/1471-2202-12-4. BMC Neurosci. 2011. PMID: 21214926 Free PMC article.
-
Traumatic brain injury and recovery mechanisms: peptide modulation of periventricular neurogenic regions by the choroid plexus-CSF nexus.J Neural Transm (Vienna). 2011 Jan;118(1):115-33. doi: 10.1007/s00702-010-0498-0. Epub 2010 Oct 10. J Neural Transm (Vienna). 2011. PMID: 20936524 Free PMC article. Review.
-
Choroid plexus epithelium and its role in neurological diseases.Front Mol Neurosci. 2022 Oct 21;15:949231. doi: 10.3389/fnmol.2022.949231. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36340696 Free PMC article. Review.
-
Experimental approaches for manipulating choroid plexus epithelial cells.Fluids Barriers CNS. 2022 May 26;19(1):36. doi: 10.1186/s12987-022-00330-2. Fluids Barriers CNS. 2022. PMID: 35619113 Free PMC article. Review.
References
-
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–5. - PubMed
-
- Bajocchi G, Feldman SH, Crystal RG, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet. 1993;3:229–34. - PubMed
-
- Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996;2:299–305. - PubMed
-
- Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Deliv Rev. 2001;46:247–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

