Aerobic fitness and physical activity levels of children born prematurely following randomization to postnatal dexamethasone
- PMID: 20732688
- PMCID: PMC2993776
- DOI: 10.1016/j.jpeds.2010.07.007
Aerobic fitness and physical activity levels of children born prematurely following randomization to postnatal dexamethasone
Abstract
Objective: To investigate the effects of postnatal dexamethasone treatment on aerobic fitness and physical activity levels in school-aged children born with very low birth weight (VLBW).
Study design: This was a follow-up study of 65 VLBW infants who participated in a randomized controlled trial of dexamethasone (DEX) to reduce ventilator dependency. Aerobic fitness was determined from peak oxygen uptake (VO(2peak)) with a cycle ergometer. Habitual physical activity was assessed by questionnaire.
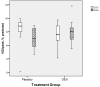
Results: A trend for a treatment with an interaction between treatment and of diagnosis of chronic lung disease (CLD) was found, with the children in the placebo group with CLD having the lowest VO(2peak) (P = .09). Reduced fitness was seen in 53% of the group treated with DEX and 48% of the group given placebo. No between-group differences in physical activity were seen. Parental reports suggested that nearly two-thirds of the children participated in < 1 hour per week of vigorous physical activity, which was explained in part by decreased large airway function (r = 0.30; P = .03).
Conclusions: We found no adverse effect of postnatal DEX on aerobic fitness or habitual physical activity at school age. However, the reduced fitness and physical activity levels emphasize the need for closer follow-up and early interventions promoting physical activity to reduce the risk of chronic disease in this at-risk population.
Copyright © 2011 Mosby, Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Hamilton BE, Martin JA, Ventura SJ. National Center for Health Statistics. National Vital Statistics Report; Hyattsville, MD: 2009. Mar 18, Births: Preliminary data for 2007. Report No.: Web release; vol 57 no 12.
-
- Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics. 2001 Jan;107(1) art-e1. - PubMed
-
- Korhonen P, Laitinen J, Hyodynmaa E, Tammela O. Respiratory outcome in school-aged, very-low-birth-weight children in the surfactant era. Acta Paediatr. 2004 Mar;93(3):316–21. - PubMed
-
- Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med. 2006 Apr 15;173(8):890–6. - PubMed