Activated PKC{delta} and PKC{epsilon} inhibit epithelial chloride secretion response to cAMP via inducing internalization of the Na+-K+-2Cl- cotransporter NKCC1
- PMID: 20732874
- PMCID: PMC2962506
- DOI: 10.1074/jbc.M110.137380
Activated PKC{delta} and PKC{epsilon} inhibit epithelial chloride secretion response to cAMP via inducing internalization of the Na+-K+-2Cl- cotransporter NKCC1
Abstract
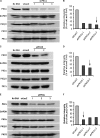
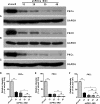
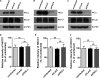
The basolateral Na(+)-K(+)-2Cl(-) cotransporter (NKCC1) is a key determinant of transepithelial chloride secretion and dysregulation of chloride secretion is a common feature of many diseases including secretory diarrhea. We have previously shown that activation of protein kinase C (PKC) markedly reduces transepithelial chloride secretion in human colonic T84 cells, which correlates with both functional inhibition and loss of the NKCC1 surface expression. In the present study, we defined the specific roles of PKC isoforms in regulating epithelial NKCC1 and chloride secretion utilizing adenoviral vectors that express shRNAs targeting human PKC isoforms (α, δ, ε) (shPKCs) or LacZ (shLacZ, non-targeting control). After 72 h of adenoviral transduction, protein levels of the PKC isoforms in shPKCs-T84 cells were decreased by ∼90% compared with the shLacZ-control. Activation of PKCs by phorbol 12-myristate 13-acetate (PMA) caused a redistribution of NKCC1 immunostaining from the basolateral membrane to intracellular vesicles in both shLacZ- and shPKCα-T84 cells, whereas the effect of PMA was not observed in shPKCδ- and shPKCε- cells. These results were further confirmed by basolateral surface biotinylation. Furthermore, activation of PKCs by PMA inhibited cAMP-stimulated chloride secretion in the uninfected, shLacZ- and shPKCα-T84 monolayers, but the inhibitory effect was significantly attenuated in shPKCδ- and shPKCε-T84 monolayers. In conclusion, the activated novel isoforms PKCδ or PKCε, but not the conventional isoform PKCα, inhibits transepithelial chloride secretion through inducing internalization of the basolateral surface NKCC1. Our study reveals that the novel PKC isoform-regulated NKCC1 surface expression plays an important role in the regulation of chloride secretion.
Figures




Similar articles
-
Dynamic regulation of Na(+)-K(+)-2Cl(-) cotransporter surface expression by PKC-{epsilon} in Cl(-)--secretory epithelia.Am J Physiol Cell Physiol. 2005 Nov;289(5):C1332-42. doi: 10.1152/ajpcell.00580.2004. Epub 2005 Jul 6. Am J Physiol Cell Physiol. 2005. PMID: 16000638
-
Phorbol 12-myristate 13-acetate-induced endocytosis of the Na-K-2Cl cotransporter in MDCK cells is associated with a clathrin-dependent pathway.Am J Physiol Cell Physiol. 2010 Jan;298(1):C85-97. doi: 10.1152/ajpcell.00118.2009. Epub 2009 Oct 28. Am J Physiol Cell Physiol. 2010. PMID: 19864322 Free PMC article.
-
Phorbol ester sequentially downregulates cAMP-regulated basolateral and apical Cl- transport pathways in T84 cells.Am J Physiol. 1993 Oct;265(4 Pt 1):C1109-17. doi: 10.1152/ajpcell.1993.265.4.C1109. Am J Physiol. 1993. PMID: 8238301
-
Molecular regulation of Na+-K+-2Cl- cotransporter (NKCC1) and epithelial chloride secretion.World J Surg. 2002 Jul;26(7):826-30. doi: 10.1007/s00268-002-4059-z. Epub 2002 Apr 15. World J Surg. 2002. PMID: 11948363 Review.
-
Na+,K+ pump and Na+-coupled ion carriers in isolated mammalian kidney epithelial cells: regulation by protein kinase C.Can J Physiol Pharmacol. 1999 May;77(5):305-19. Can J Physiol Pharmacol. 1999. PMID: 10535680 Review.
Cited by
-
Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport.Physiol Rev. 2012 Oct;92(4):1577-617. doi: 10.1152/physrev.00009.2012. Physiol Rev. 2012. PMID: 23073627 Free PMC article. Review.
-
Roles of PKC and phospho-adducin in transepithelial fluid secretion by Malpighian tubules of the yellow fever mosquito.Tissue Barriers. 2013 Jan 1;1(1):e23120. doi: 10.4161/tisb.23120. Tissue Barriers. 2013. PMID: 24062972 Free PMC article.
-
Short-term regulation of murine colonic NBCe1-B (electrogenic Na+/HCO3(-) cotransporter) membrane expression and activity by protein kinase C.PLoS One. 2014 Mar 18;9(3):e92275. doi: 10.1371/journal.pone.0092275. eCollection 2014. PLoS One. 2014. PMID: 24642792 Free PMC article.
-
Synergistic Effects between mTOR Complex 1/2 and Glycolysis Inhibitors in Non-Small-Cell Lung Carcinoma Cells.PLoS One. 2015 Jul 15;10(7):e0132880. doi: 10.1371/journal.pone.0132880. eCollection 2015. PLoS One. 2015. PMID: 26176608 Free PMC article.
-
Differential regulation of cyclin D1 expression by protein kinase C α and ϵ signaling in intestinal epithelial cells.J Biol Chem. 2014 Aug 8;289(32):22268-83. doi: 10.1074/jbc.M114.571554. Epub 2014 Jun 9. J Biol Chem. 2014. PMID: 24914206 Free PMC article.
References
-
- Russell J. M. (2000) Physiol. Rev. 80, 211–276 - PubMed
-
- Haas M., Forbush B., 3rd (2000) Annu. Rev. Physiol. 62, 515–534 - PubMed
-
- Lebenthal E., Duffey M. E. (1990) Textbook of Secretory Diarrhea, Raven Press, New York
-
- Halm D. R., Raymond F. A. (1990) in Textbook of Secretory Diarrhea (Lebenthal E., Duffey M. E. eds) pp. 47–58, Raven Press, New York
-
- Matthews J. B. (2002) World J. Surg. 26, 826–830 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

