Testicular expression of Adora3i2 in Adora3 knockout mice reveals a role of mouse A3Ri2 and human A3Ri3 adenosine receptors in sperm
- PMID: 20732875
- PMCID: PMC2962464
- DOI: 10.1074/jbc.M110.156075
Testicular expression of Adora3i2 in Adora3 knockout mice reveals a role of mouse A3Ri2 and human A3Ri3 adenosine receptors in sperm
Abstract
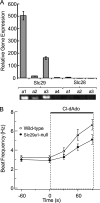
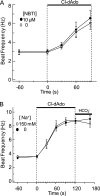
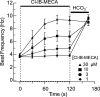
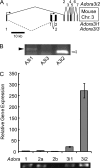
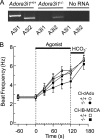
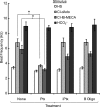
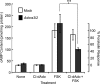
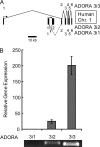
Adenosine is a candidate modulator of sperm motility in the female reproductive tract that increases sperm flagellar beat frequency in vitro. Past work suggested that this acceleration may involve equilibrative (ENT) and concentrative (CNT) nucleoside transporters. Here we show that Slc29a1 (ENT-1) is the predominant nucleoside transporter expressed in the mouse testis. Unexpectedly, the beat of Slc29a1-null sperm still accelerates in response to 2-chloro-2'-deoxyadenosine (Cl-dAdo). Moreover, in wild-type sperm neither blockade of CNTs by removal of external Na(+), nor inhibition of ENTs with nitrobenzylthioionosine, prevents acceleration of the sperm beat by Cl-dAdo. In contrast, pertussis toxin produces strong blockade, indicating involvement of a Gα(i/o)-coupled adenosine receptor. Although agonists selective for adenosine receptors A1R, A2aR, and A2bR are ineffective, A3R-selective agonists Cl-IB-MECA and IB-MECA do accelerate the beat. Consistent with this pharmacological profile, the predominant Adora transcripts in the testis are products of the nested Adora3i1 and Adora3i2 genes. Surprisingly, Cl-IB-MECA and Cl-dAdo still accelerate the beat of Adora3i1-null sperm indicating that the remaining Adora3i2 transcript produces an A3R that functions in sperm. When cloned Adora3i2 is heterologously expressed in tsA-201 cells, Cl-dAdo decreases forskolin-evoked accumulation of cAMP, indicating that Adora3i2 specifies a functional A3Ri2 adenosine receptor that couples through Gα(i). Database mining reveals that mouse Adora3i2 is expressed primarily in testis, almost exclusively in spermatids. Expression of the orthologous ADORA3i3 transcript also is most prominent in human testis; presumably producing an A3Ri3 receptor that is functional in sperm and that may be a target for development of male-directed contraceptives.
Figures
Similar articles
-
Adenosine and catecholamine agonists speed the flagellar beat of mammalian sperm by a non-receptor-mediated mechanism.Biol Reprod. 2007 Dec;77(6):960-9. doi: 10.1095/biolreprod.107.062562. Epub 2007 Aug 29. Biol Reprod. 2007. PMID: 17761644
-
Activation of adenosine A3 receptors reduces ischemic brain injury in rodents.J Neurosci Res. 2006 Dec;84(8):1848-55. doi: 10.1002/jnr.21071. J Neurosci Res. 2006. PMID: 17016854
-
Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility.J Cell Sci. 2004 Nov 15;117(Pt 24):5835-45. doi: 10.1242/jcs.01507. Epub 2004 Nov 2. J Cell Sci. 2004. PMID: 15522887
-
The role of the adenosinergic system in lung fibrosis.Pharmacol Res. 2013 Oct;76:182-9. doi: 10.1016/j.phrs.2013.08.004. Epub 2013 Aug 28. Pharmacol Res. 2013. PMID: 23994158 Review.
-
Adenosine receptors as potential targets in melanoma.Pharmacol Res. 2013 Oct;76:34-40. doi: 10.1016/j.phrs.2013.07.002. Epub 2013 Jul 13. Pharmacol Res. 2013. PMID: 23856527 Review.
Cited by
-
Ability of CP-532,903 to protect mouse hearts from ischemia/reperfusion injury is dependent on expression of A3 adenosine receptors in cardiomyoyctes.Biochem Pharmacol. 2019 May;163:21-31. doi: 10.1016/j.bcp.2019.01.022. Epub 2019 Jan 30. Biochem Pharmacol. 2019. PMID: 30710517 Free PMC article.
-
New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors.Purinergic Signal. 2012 Sep;8(3):587-98. doi: 10.1007/s11302-012-9311-x. Epub 2012 Apr 15. Purinergic Signal. 2012. PMID: 22528684 Free PMC article. Review.
-
Single-cell Motility Analysis of Tethered Human Spermatozoa.Bio Protoc. 2019 Mar 5;9(5):e3182. doi: 10.21769/BioProtoc.3182. Bio Protoc. 2019. PMID: 31032381 Free PMC article.
-
Episodic rolling and transient attachments create diversity in sperm swimming behavior.BMC Biol. 2014 Aug 16;12:67. doi: 10.1186/s12915-014-0067-3. BMC Biol. 2014. PMID: 25182562 Free PMC article.
-
Leu128(3.43) (l128) and Val247(6.40) (V247) of CXCR1 are critical amino acid residues for g protein coupling and receptor activation.PLoS One. 2012;7(8):e42765. doi: 10.1371/journal.pone.0042765. Epub 2012 Aug 24. PLoS One. 2012. PMID: 22936990 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

