Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids
- PMID: 20732876
- PMCID: PMC2963419
- DOI: 10.1074/jbc.M110.118406
Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids
Abstract
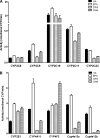
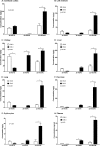
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) protect against cardiovascular disease by largely unknown mechanisms. We tested the hypothesis that EPA and DHA may compete with arachidonic acid (AA) for the conversion by cytochrome P450 (CYP) enzymes, resulting in the formation of alternative, physiologically active, metabolites. Renal and hepatic microsomes, as well as various CYP isoforms, displayed equal or elevated activities when metabolizing EPA or DHA instead of AA. CYP2C/2J isoforms converting AA to epoxyeicosatrienoic acids (EETs) preferentially epoxidized the ω-3 double bond and thereby produced 17,18-epoxyeicosatetraenoic (17,18-EEQ) and 19,20-epoxydocosapentaenoic acid (19,20-EDP) from EPA and DHA. We found that these ω-3 epoxides are highly active as antiarrhythmic agents, suppressing the Ca(2+)-induced increased rate of spontaneous beating of neonatal rat cardiomyocytes, at low nanomolar concentrations. CYP4A/4F isoforms ω-hydroxylating AA were less regioselective toward EPA and DHA, catalyzing predominantly ω- and ω minus 1 hydroxylation. Rats given dietary EPA/DHA supplementation exhibited substantial replacement of AA by EPA and DHA in membrane phospholipids in plasma, heart, kidney, liver, lung, and pancreas, with less pronounced changes in the brain. The changes in fatty acids were accompanied by concomitant changes in endogenous CYP metabolite profiles (e.g. altering the EET/EEQ/EDP ratio from 87:0:13 to 27:18:55 in the heart). These results demonstrate that CYP enzymes efficiently convert EPA and DHA to novel epoxy and hydroxy metabolites that could mediate some of the beneficial cardiovascular effects of dietary ω-3 fatty acids.
Figures





References
-
- Capdevila J. H., Falck J. R. (2002) Prostaglandins Other Lipid Mediat. 68, 325–344 - PubMed
-
- Zeldin D. C. (2001) J. Biol. Chem. 276, 36059–36062 - PubMed
-
- Kroetz D. L., Xu F. (2005) Annu. Rev. Pharmacol. Toxicol. 45, 413–438 - PubMed
-
- Campbell W. B., Falck J. R. (2007) Hypertension 49, 590–596 - PubMed
-
- Fleming I. (2008) Trends Cardiovasc. Med. 18, 20–25 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

