Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels
- PMID: 20732880
- PMCID: PMC2993909
- DOI: 10.1093/jxb/erq257
Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels
Abstract
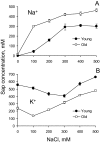
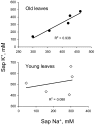
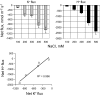
Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) were studied by exposing plants to six salinity levels (0-500 mM NaCl range) for 70 d. Salt stress was administered either by pre-mixing of the calculated amount of NaCl with the potting mix before seeds were planted or by the gradual increase of NaCl levels in the irrigation water. For both methods, the optimal plant growth and biomass was achieved between 100 mM and 200 mM NaCl, suggesting that quinoa possess a very efficient system to adjust osmotically for abrupt increases in NaCl stress. Up to 95% of osmotic adjustment in old leaves and between 80% and 85% of osmotic adjustment in young leaves was achieved by means of accumulation of inorganic ions (Na(+), K(+), and Cl(-)) at these NaCl levels, whilst the contribution of organic osmolytes was very limited. Consistently higher K(+) and lower Na(+) levels were found in young, as compared with old leaves, for all salinity treatments. The shoot sap K(+) progressively increased with increased salinity in old leaves; this is interpreted as evidence for the important role of free K(+) in leaf osmotic adjustment under saline conditions. A 5-fold increase in salinity level (from 100 mM to 500 mM) resulted in only a 50% increase in the sap Na(+) content, suggesting either a very strict control of xylem Na(+) loading or an efficient Na(+) removal from leaves. A very strong correlation between NaCl-induced K(+) and H(+) fluxes was observed in quinoa root, suggesting that a rapid NaCl-induced activation of H(+)-ATPase is needed to restore otherwise depolarized membrane potential and prevent further K(+) leak from the cytosol. Taken together, this work emphasizes the role of inorganic ions for osmotic adjustment in halophytes and calls for more in-depth studies of the mechanisms of vacuolar Na(+) sequestration, control of Na(+) and K(+) xylem loading, and their transport to the shoot.
Figures





Similar articles
-
The combined effect of Cr(III) and NaCl determines changes in metal uptake, nutrient content, and gene expression in quinoa (Chenopodium quinoa Willd.).Ecotoxicol Environ Saf. 2020 Apr 15;193:110345. doi: 10.1016/j.ecoenv.2020.110345. Epub 2020 Feb 21. Ecotoxicol Environ Saf. 2020. PMID: 32092578
-
Rapid regulation of the plasma membrane H⁺-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa.Ann Bot. 2015 Feb;115(3):481-94. doi: 10.1093/aob/mcu219. Epub 2014 Dec 2. Ann Bot. 2015. PMID: 25471095 Free PMC article.
-
Comparative physiological and biochemical mechanisms of salt tolerance in five contrasting highland quinoa cultivars.BMC Plant Biol. 2020 Feb 12;20(1):70. doi: 10.1186/s12870-020-2279-8. BMC Plant Biol. 2020. PMID: 32050903 Free PMC article.
-
Osmotic adjustment and energy limitations to plant growth in saline soil.New Phytol. 2020 Feb;225(3):1091-1096. doi: 10.1111/nph.15862. Epub 2019 May 21. New Phytol. 2020. PMID: 31006123 Review.
-
Salinity tolerance in halophytes.New Phytol. 2008;179(4):945-963. doi: 10.1111/j.1469-8137.2008.02531.x. Epub 2008 Jun 28. New Phytol. 2008. PMID: 18565144 Review.
Cited by
-
Combined transcriptome and metabolome analysis of the resistance mechanism of quinoa seedlings to Spodoptera exigua.Front Plant Sci. 2022 Jul 28;13:931145. doi: 10.3389/fpls.2022.931145. eCollection 2022. Front Plant Sci. 2022. PMID: 35968105 Free PMC article.
-
A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value.Cell Res. 2017 Nov;27(11):1327-1340. doi: 10.1038/cr.2017.124. Epub 2017 Oct 10. Cell Res. 2017. PMID: 28994416 Free PMC article.
-
Diffusive and Metabolic Constraints to Photosynthesis in Quinoa during Drought and Salt Stress.Plants (Basel). 2017 Oct 17;6(4):49. doi: 10.3390/plants6040049. Plants (Basel). 2017. PMID: 29039809 Free PMC article.
-
Single-cell transcriptomic analysis reveals the developmental trajectory and transcriptional regulatory networks of quinoa salt bladders.Stress Biol. 2024 Nov 13;4(1):47. doi: 10.1007/s44154-024-00189-3. Stress Biol. 2024. PMID: 39532803 Free PMC article.
-
The Salinity Survival Strategy of Chenopodium quinoa: Investigating Microbial Community Shifts and Nitrogen Cycling in Saline Soils.Microorganisms. 2023 Nov 21;11(12):2829. doi: 10.3390/microorganisms11122829. Microorganisms. 2023. PMID: 38137973 Free PMC article.
References
-
- Beilby MJ, Shepherd VA. Modeling the current–voltage characteristics of charophyte membranes. II. The effect of salinity on membranes of Lamprothamnium papulosum. Journal of Membrane Biology. 2001;181:77–89. - PubMed
-
- Bisson MA, Kirst GO. Lamprothamnium, a euryhaline charophyte. 1. Osmotic relations and membrane-potential at steady-state. Journal of Experimental Botany. 1980;31:1223–1235.
-
- Blumwald E. Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology. 2000;12:431–434. - PubMed
-
- Bohnert HJ, Shen B. Transformation and compatible solutes. Scientia Horticulturae. 1999;78:237–260.
-
- Chen THH, Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Current Opinion in Plant Biology. 2002;5:250–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

