Expression profiling of cell cycle genes reveals key facilitators of cell production during carpel development, fruit set, and fruit growth in apple (Malusxdomestica Borkh.)
- PMID: 20732881
- PMCID: PMC2993910
- DOI: 10.1093/jxb/erq258
Expression profiling of cell cycle genes reveals key facilitators of cell production during carpel development, fruit set, and fruit growth in apple (Malusxdomestica Borkh.)
Abstract
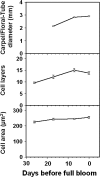
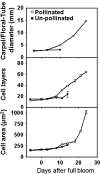
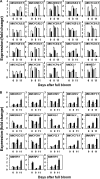
Cell production is an essential facilitator of fruit growth and development. Cell production during carpel/floral-tube growth, fruit set, and fruit growth, and its regulation by cell cycle genes were investigated in apple (Malus×domestica Borkh.). Cell production was inhibited during late carpel/floral-tube development, resulting in growth arrest before bloom. Fruit set re-activated cell production between 8 d and 11 d after full bloom (DAFB) and triggered fruit growth. The early phase of fruit growth involved rapid cell production followed by exit from cell proliferation at ∼24 DAFB. Seventy-one cell cycle genes were identified, and expression of 59 genes was investigated using quantitative RT-PCR. Changes in expression of 19 genes were consistently associated with transitions in cell production during carpel/floral-tube growth, fruit set, and fruit growth. Fourteen genes, including B-type cyclin-dependent kinases (CDKs) and A2-, B1-, and B2-type cyclins, were positively associated with cell production, suggesting that availability of G2/M phase regulators of the cell cycle is limiting for cell proliferation. Enhanced expression of five genes including that of the putative CDK inhibitors, MdKRP4 and MdKRP5, was associated with reduced cell production. Exit from cell proliferation at G0/G1 during fruit growth was facilitated by multiple mechanisms including down-regulation of putative regulators of G1/S and G2/M phase progression and up-regulation of KRP genes. Interestingly, two CDKA genes and several CDK-activating factors were up-regulated during this period, suggesting functions for these genes in mediating exit from cell proliferation at G0/G1. Together, the data indicate that cell cycle genes are important facilitators of cell production during apple fruit development.
Figures



Similar articles
-
The AINTEGUMENTA genes, MdANT1 and MdANT2, are associated with the regulation of cell production during fruit growth in apple (Malus × domestica Borkh.).BMC Plant Biol. 2012 Jun 25;12:98. doi: 10.1186/1471-2229-12-98. BMC Plant Biol. 2012. PMID: 22731507 Free PMC article.
-
Increase in fruit size of a spontaneous mutant of 'Gala' apple (Malus x domestica Borkh.) is facilitated by altered cell production and enhanced cell size.J Exp Bot. 2010 Jun;61(11):3003-13. doi: 10.1093/jxb/erq134. Epub 2010 May 19. J Exp Bot. 2010. PMID: 20484321 Free PMC article.
-
Differential response of cell-cycle and cell-expansion regulators to heat stress in apple (Malus domestica) fruitlets.Plant Sci. 2015 Apr;233:82-94. doi: 10.1016/j.plantsci.2015.01.005. Epub 2015 Jan 14. Plant Sci. 2015. PMID: 25711816
-
Research progress of fruit color development in apple (Malus domestica Borkh.).Plant Physiol Biochem. 2021 May;162:267-279. doi: 10.1016/j.plaphy.2021.02.033. Epub 2021 Mar 4. Plant Physiol Biochem. 2021. PMID: 33711720 Review.
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
Cited by
-
Histological, hormonal and transcriptomic reveal the changes upon gibberellin-induced parthenocarpy in pear fruit.Hortic Res. 2018 Jan 3;5:1. doi: 10.1038/s41438-017-0012-z. eCollection 2018. Hortic Res. 2018. PMID: 29423231 Free PMC article.
-
Comparative Transcriptome and Microscopy Analyses Provide Insights into Flat Shape Formation in Peach (Prunus persica).Front Plant Sci. 2018 Jan 4;8:2215. doi: 10.3389/fpls.2017.02215. eCollection 2017. Front Plant Sci. 2018. PMID: 29354151 Free PMC article.
-
The AINTEGUMENTA genes, MdANT1 and MdANT2, are associated with the regulation of cell production during fruit growth in apple (Malus × domestica Borkh.).BMC Plant Biol. 2012 Jun 25;12:98. doi: 10.1186/1471-2229-12-98. BMC Plant Biol. 2012. PMID: 22731507 Free PMC article.
-
Staying in touch: how highly specialised moth pollinators track host plant phenology in unpredictable climates.BMC Ecol Evol. 2021 Aug 24;21(1):161. doi: 10.1186/s12862-021-01889-4. BMC Ecol Evol. 2021. PMID: 34429068 Free PMC article.
-
Higher growth of the apple (Malus × domestica Borkh.) fruit cortex is supported by resource intensive metabolism during early development.BMC Plant Biol. 2020 Feb 13;20(1):75. doi: 10.1186/s12870-020-2280-2. BMC Plant Biol. 2020. PMID: 32054442 Free PMC article.
References
-
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GTS, Genschik P. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Current Biology. 2009;19:1188–1193. - PubMed
-
- Arias EE, Walter JC. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes and Development. 2007;21:497–518. - PubMed
-
- Baldet P, Hernould M, Laporte F, Mounet F, Just D, Mouras A, Chevalier C, Rothan C. The expression of cell proliferation-related genes in early developing flowers is affected by a fruit load reduction in tomato plants. Journal of Experimental Botany. 2006;57:961–970. - PubMed
-
- Berlyn GP, Miksche JP. Botanical microtechnique and cytochemistry. Ames, IA: Iowa State University Press; 1976. Fixation and storage. 24–34.

