Oligomerization of Cavbeta subunits is an essential correlate of Ca2+ channel activity
- PMID: 20732952
- PMCID: PMC2992367
- DOI: 10.1096/fj.10-165381
Oligomerization of Cavbeta subunits is an essential correlate of Ca2+ channel activity
Abstract
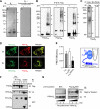
Voltage-gated calcium channels conduct Ca(2+) ions in response to membrane depolarization. The resulting transient increase in cytoplasmic free calcium concentration is a critical trigger for the initiation of such vital responses as muscle contraction and transcription. L-type Ca(v)1.2 calcium channels are complexes of the pore-forming α(1C) subunit associated with cytosolic Ca(v)β subunits. All major Ca(v)βs share a highly homologous membrane associated guanylate kinase-like (MAGUK) domain that binds to α(1C) at the α-interaction domain (AID), a short motif in the linker between transmembrane repeats I and II. In this study we show that Ca(v)β subunits form multimolecular homo- and heterooligomeric complexes in human vascular smooth muscle cells expressing native calcium channels and in Cos7 cells expressing recombinant Ca(v)1.2 channel subunits. Ca(v)βs oligomerize at the α(1C) subunits residing in the plasma membrane and bind to the AID. However, Ca(v)β oligomerization occurs independently on the association with α(1C). Molecular structures responsible for Ca(v)β oligomerization reside in 3 regions of the guanylate kinase subdomain of MAGUK. An augmentation of Ca(v)β homooligomerization significantly increases the calcium current density, while heterooligomerization may also change the voltage-dependence and inactivation kinetics of the channel. Thus, oligomerization of Ca(v)β subunits represents a novel and essential aspect of calcium channel regulation.
Figures

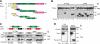
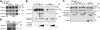

References
-
- Westenbroek R. E., Ahlijanian M. K., Catterall W. A. (1990) Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature 347, 281–284 - PubMed
-
- Catterall W. A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

