α6ß2* and α4ß2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: relevance to Parkinson's disease
- PMID: 20732972
- PMCID: PMC2981368
- DOI: 10.1124/mol.110.067561
α6ß2* and α4ß2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: relevance to Parkinson's disease
Abstract
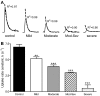
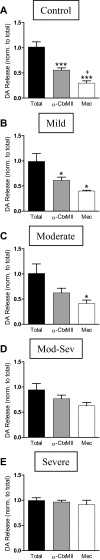
Nicotinic receptors (nAChRs) are important modulators of dopaminergic transmission in striatum, a region critical to Parkinson's disease. The nAChRs mainly involved are the α6β2* and α4β2* subtypes. Lesion studies show that the α6β2* receptor is decreased to a much greater extent with nigrostriatal damage than the α4β2* subtype raising the question whether this latter nAChR population is more important with increased nigrostriatal damage. To address this, we investigated the effect of varying nigrostriatal damage on α6β2* and α4β2* receptor-modulated dopamine signaling using cyclic voltammetry. This approach offers the advantage that changes in dopamine release can be observed under different neuronal firing conditions. Total single-pulse-evoked dopamine release decreased in direct proportion to declines in the dopamine transporter and dopamine uptake. We next used α-conotoxinMII and mecamylamine to understand the role of the α4β2* and α6β2* subtypes in release. Single-pulse-stimulated α6β2* and α4β2* receptor dopamine release decreased to a similar extent with increasing nigrostriatal damage, indicating that both subtypes contribute to the control of dopaminergic transmission with lesioning. Total burst-stimulated dopamine release also decreased proportionately with nigrostriatal damage. However, the role of the α4β2* and α6β2* nAChRs varied with different degrees of lesioning, suggesting that the two subtypes play a unique function with burst firing, with a somewhat more prominent and possibly more selective role for the α6β2* subtype. These data have important therapeutic implications because they suggest that drugs directed to both α4β2* and α6β2* nAChRs may be useful in the treatment of neurological disorders such as Parkinson's disease.
Figures
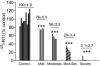



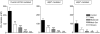
References
-
- Artymyshyn R, Smith A, Wolfe BB. (1990) The use of 3H standards in 125I autoradiography. J Neurosci Methods 32:185–192 - PubMed
-
- Bergstrom BP, Schertz KE, Weirick T, Nafziger B, Takacs SA, Lopes KO, Massa KJ, Walker QD, Garris PA. (2001) Partial, graded losses of dopamine terminals in the rat caudate-putamen: an animal model for the study of compensatory adaptation in preclinical parkinsonism. J Neurosci Methods 106:15–28 - PubMed
-
- Bordia T, Campos C, Huang L, Quik M. (2008) Continuous and intermittent nicotine treatment reduces l-3,4-dihydroxyphenylalanine (l-DOPA)-induced dyskinesias in a rat model of Parkinson's disease. J Pharmacol Exp Ther 327:239–247 - PubMed
-
- Bordia T, Grady SR, McIntosh JM, Quik M. (2007) Nigrostriatal damage preferentially decreases a subpopulation of alpha6beta2* nAChRs in mouse, monkey, and Parkinson's disease striatum. Mol Pharmacol 72:52–61 - PubMed
-
- Cenci MA, Lundblad M. (2007) Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson's disease in rats and mice. Curr Protoc Neurosci Chapter 9:Unit 9.25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

