ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis
- PMID: 20732973
- PMCID: PMC2949020
- DOI: 10.1104/pp.110.161968
ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis
Abstract
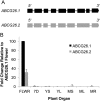
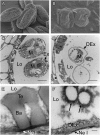
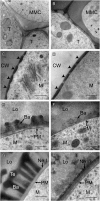
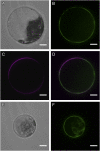
The highly resistant biopolymer, sporopollenin, gives the outer wall (exine) of spores and pollen grains their unparalleled strength, shielding these structures from terrestrial stresses. Despite a limited understanding of the composition of sporopollenin, it appears that the synthesis of sporopollenin occurs in the tapetum and requires the transport of one or more sporopollenin constituents to the surface of developing microspores. Here, we describe ABCG26, a member of the ATP-binding cassette (ABC) transporter superfamily, which is required for pollen exine formation in Arabidopsis (Arabidopsis thaliana). abcg26 mutants are severely reduced in fertility, with most siliques failing to produce seeds by self-fertilization and mature anthers failing to release pollen. Transmission electron microscopy analyses revealed an absence of an exine wall on abcg26-1 mutant microspores. Phenotypic abnormalities in pollen wall formation were first apparent in early uninucleate microspores as a lack of exine formation and sporopollenin deposition. Additionally, the highest levels of ABCG26 mRNA were in the tapetum, during early pollen wall formation, sporopollenin biosynthesis, and sporopollenin deposition. Accumulations resembling the trilamellar lipidic coils in the abcg11 and abcg12 mutants defective in cuticular wax export were observed in the anther locules of abcg26 mutants. A yellow fluorescent protein-ABCG26 protein was localized to the endoplasmic reticulum and plasma membrane. Our results show that ABCG26 plays a critical role in exine formation and pollen development and are consistent with a model by which ABCG26 transports sporopollenin precursors across the tapetum plasma membrane into the locule for polymerization on developing microspore walls.
Figures





References
-
- Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, Stiekema WJ, Scott R, Pereira A. (1997) The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J 12: 615–623 - PubMed
-
- Ahlers F, Bubert H, Steuernagel S, Wiermann R. (2000) The nature of oxygen in sporopollenin from the pollen of Typha angustifolia L. Z Naturforsch C 55: 129–136 - PubMed
-
- Ahlers F, Lambert J, Wiermann R. (2003) Acetylation and silylation of piperidine solubilized sporopollenin from pollen of Typha angustifolia L. Z Naturforsch C 58: 807–811 - PubMed
-
- Ahlers F, Thoma I, Lambert J, Kuckuk R, Wiermann R. (1999) 1H NMR analysis of sporopollenin from Typha angustifolia. Phytochemistry 50: 1095–1098
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

