Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor
- PMID: 20733005
- PMCID: PMC2950554
- DOI: 10.1128/MCB.00180-10
Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor
Abstract
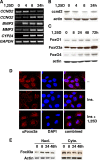
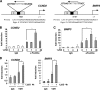
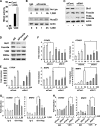
Hormonal vitamin D, 1,25-dihydroxyvitamin D (1,25D), signals through the nuclear vitamin D receptor (VDR). 1,25D regulates cell proliferation and differentiation and has been identified as a cancer chemopreventive agent. FoxO proteins are transcription factors that control cell proliferation and survival. They function as tumor suppressors and are associated with longevity in several organisms. Accumulating data have revealed that 1,25D and FoxO proteins regulate similarly common target genes. We show here that the ligand-bound VDR regulates the posttranslational modification and function of FoxO proteins. 1,25D treatment enhances binding of FoxO3a and FoxO4 within 4 h to promoters of FoxO target genes and blocks mitogen-induced FoxO protein nuclear export. The VDR associates directly with FoxO proteins and regulators, the sirtuin 1 (Sirt1) class III histone deacetylase (HDAC), and protein phosphatase 1. In addition, phosphatase activity and trichostatin A-resistant HDAC activity coimmunoprecipitate with the VDR. 1,25D treatment rapidly (in <4 h) induces FoxO deacetylation and dephosphorylation, consistent with activation. In contrast, ablation of VDR expression enhances FoxO3a phosphorylation, as does knockdown of Sirt1, consistent with the coupling of FoxO acetylation and phosphorylation. 1,25D regulation of common VDR/FoxO target genes is attenuated by blockade of phosphatase activity or by small interfering RNA (siRNA)-mediated knockdown of Sirt1 or FoxO protein expression. Finally, 1,25D-dependent cell cycle arrest is blocked in FoxO3a-deficient cells, indicating that FoxO proteins are key downstream mediators of the antiproliferative actions of 1,25D. These studies link 1,25D signaling through the VDR directly to Sirt1 and FoxO function and provide a molecular basis for the cancer chemopreventive actions of 1,25D.
Figures


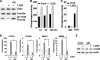

References
-
- Accili, D., and K. C. Arden. 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421-426. - PubMed
-
- Adorini, L. 2005. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell. Immunol. 233:115-124. - PubMed
-
- Akutsu, N., R. Lin, Y. Bastien, A. Bestawros, D. J. Enepekides, M. J. Black, and J. H. White. 2001. Regulation of gene expression by 1{{alpha}},25-dihydroxyvitamin D3 and its analog EB1089 under growth-inhibitory conditions in squamous carcinoma cells. Mol. Endocrinol. 15:1127-1139. - PubMed
-
- An, B.-S., D. M. Selva, G. L. Hammond, A. Rivero-Muller, N. Rahman, and P. C. K. Leung. 2006. Steroid receptor coactivator-3 is required for progesterone receptor trans-activation of target genes in response to gonadotropin-releasing hormone treatment of pituitary cells. J. Biol. Chem. 281:20817-20824. - PubMed
-
- Arden, K. C. 2007. FoxOs in tumor suppression and stem cell maintenance. Cell 128:235-237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
