CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses
- PMID: 20733031
- PMCID: PMC2931170
- DOI: 10.1084/jem.20100098
CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses
Abstract
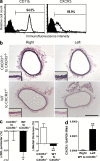
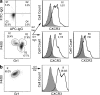
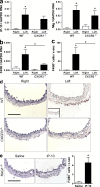
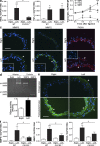
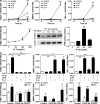
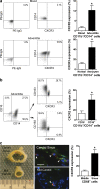
Sustained changes in blood flow modulate the size of conduit arteries through structural alterations of the vessel wall that are dependent on the transient accumulation and activation of perivascular macrophages. The leukocytic infiltrate appears to be confined to the adventitia, is responsible for medial remodeling, and resolves once hemodynamic stresses have normalized without obvious intimal changes. We report that inward remodeling of the mouse common carotid artery after ligation of the ipsilateral external carotid artery is dependent on the chemokine receptor CXCR3. Wild-type myeloid cells restored flow-mediated vascular remodeling in CXCR3-deficient recipients, adventitia-infiltrating macrophages of Gr1(low) resident phenotype expressed CXCR3, the perivascular accumulation of macrophages was dependent on CXCR3 signaling, and the CXCR3 ligand IP-10 was sufficient to recruit monocytes to the adventitia. CXCR3 also contributed to selective features of macrophage activation required for extracellular matrix turnover, such as production of the transglutaminase factor XIII A subunit. Human adventitial macrophages displaying a CD14(+)/CD16(+) resident phenotype, but not circulating monocytes, expressed CXCR3, and such cells were more frequent at sites of disturbed flow. Our observations reveal a CXCR3-dependent accumulation and activation of perivascular macrophages as a necessary step in homeostatic arterial remodeling triggered by hemodynamic stress in mice and possibly in humans as well.
Figures



Similar articles
-
MyD88-dependent, superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries.J Exp Med. 2008 Dec 22;205(13):3159-71. doi: 10.1084/jem.20081298. Epub 2008 Dec 8. J Exp Med. 2008. PMID: 19064699 Free PMC article.
-
CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model.Immunology. 2014 Sep;143(1):109-19. doi: 10.1111/imm.12293. Immunology. 2014. PMID: 24679047 Free PMC article.
-
Impairment in postischemic neovascularization in mice lacking the CXC chemokine receptor 3.Circ Res. 2005 Mar 18;96(5):576-82. doi: 10.1161/01.RES.0000159389.55544.20. Epub 2005 Feb 17. Circ Res. 2005. PMID: 15718500
-
CXCR3+ monocytes/macrophages are required for establishment of pulmonary metastases.Sci Rep. 2017 Mar 30;7:45593. doi: 10.1038/srep45593. Sci Rep. 2017. PMID: 28358049 Free PMC article.
-
The adventitia in arterial development, remodeling, and hypertension.Biochem Pharmacol. 2022 Nov;205:115259. doi: 10.1016/j.bcp.2022.115259. Epub 2022 Sep 21. Biochem Pharmacol. 2022. PMID: 36150432 Review.
Cited by
-
Exploration of the metabolomic mechanisms of postmenopausal hypertension induced by low estrogen state.Elife. 2025 Jan 16;13:RP101701. doi: 10.7554/eLife.101701. Elife. 2025. PMID: 39817721 Free PMC article.
-
Excessive adventitial stress drives inflammation-mediated fibrosis in hypertensive aortic remodelling in mice.J R Soc Interface. 2021 Jul;18(180):20210336. doi: 10.1098/rsif.2021.0336. Epub 2021 Jul 28. J R Soc Interface. 2021. PMID: 34314650 Free PMC article.
-
dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury.Cell Death Differ. 2022 Dec;29(12):2487-2502. doi: 10.1038/s41418-022-01033-9. Epub 2022 Jun 23. Cell Death Differ. 2022. PMID: 35739254 Free PMC article.
-
Age-related neointimal hyperplasia is associated with monocyte infiltration after balloon angioplasty.J Gerontol A Biol Sci Med Sci. 2012 Feb;67(2):109-17. doi: 10.1093/gerona/glr190. Epub 2011 Oct 19. J Gerontol A Biol Sci Med Sci. 2012. PMID: 22016364 Free PMC article.
-
CXCR3 modulates obesity-induced visceral adipose inflammation and systemic insulin resistance.Obesity (Silver Spring). 2014 May;22(5):1264-74. doi: 10.1002/oby.20642. Epub 2014 Mar 27. Obesity (Silver Spring). 2014. PMID: 24124129 Free PMC article.
References
-
- Bakker E.N., Pistea A., Spaan J.A., Rolf T., de Vries C.J., van Rooijen N., Candi E., VanBavel E. 2006. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ. Res. 99:86–92 10.1161/01.RES.0000229657.83816.a7 - DOI - PubMed
-
- Bassiouny H.S., Song R.H., Hong X.F., Singh A., Kocharyan H., Glagov S. 1998. Flow regulation of 72-kD collagenase IV (MMP-2) after experimental arterial injury. Circulation. 98:157–163 - PubMed
-
- Baumbach G.L., Heistad D.D. 1989. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 13:968–972 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

