PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps
- PMID: 20733033
- PMCID: PMC2931169
- DOI: 10.1084/jem.20100239
PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps
Abstract
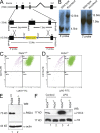
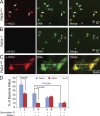
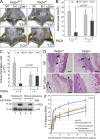
Neutrophils trap and kill bacteria by forming highly decondensed chromatin structures, termed neutrophil extracellular traps (NETs). We previously reported that histone hypercitrullination catalyzed by peptidylarginine deiminase 4 (PAD4) correlates with chromatin decondensation during NET formation. However, the role of PAD4 in NET-mediated bacterial trapping and killing has not been tested. Here, we use PAD4 knockout mice to show that PAD4 is essential for NET-mediated antibacterial function. Unlike PAD4(+/+) neutrophils, PAD4(-/-) neutrophils cannot form NETs after stimulation with chemokines or incubation with bacteria, and are deficient in bacterial killing by NETs. In a mouse infectious disease model of necrotizing fasciitis, PAD4(-/-) mice are more susceptible to bacterial infection than PAD4(+/+) mice due to a lack of NET formation. Moreover, we found that citrullination decreased the bacterial killing activity of histones and nucleosomes, which suggests that PAD4 mainly plays a role in chromatin decondensation to form NETs instead of increasing histone-mediated bacterial killing. Our results define a role for histone hypercitrullination in innate immunity during bacterial infection.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

