Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis
- PMID: 20733043
- PMCID: PMC2976143
- DOI: 10.1128/AAC.00177-10
Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis
Abstract
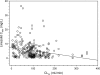
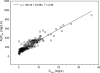
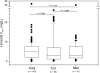
The objective of the present retrospective observational study carried out in patients receiving a standard dosage of linezolid and undergoing routine therapeutic drug monitoring (TDM) was to assess the interindividual variability in plasma exposure, to identify the prevalence of attainment of optimal pharmacodynamics, and to define if an intensive program of TDM may be warranted in some categories of patients. Linezolid plasma concentrations (trough [C(min)] and peak [C(max)] levels) were analyzed by means of a high-performance liquid chromatography (HPLC) method, and daily drug exposure was estimated (daily area under the plasma concentration-time curve [AUC(24)]). The final database included 280 C(min) and 223 C(max) measurements performed in 92 patients who were treated with the fixed 600-mg dose every 12 h (q12h) intravenously (n = 58) or orally (n = 34). A wide variability was observed (median values [interquartile range]: 3.80 mg/liter [1.75 to 7.53 mg/liter] for C(min), 14.70 mg/liter [10.57 to 19.64] for C(max), and 196.08 mg·h/liter [144.02 to 312.10 mg·h/liter] for estimated AUC(24)). Linezolid C(min) was linearly correlated with estimated AUC(24) (r(2) = 0.85). Optimal pharmacodynamic target attainment (defined as C(min) of ≥2 mg/liter and/or AUC(24)/MIC(90) ratio of >80) was obtained in about 60 to 70% of cases, but potential overexposure (defined as C(min) of ≥10 mg/liter and/or AUC(24) of ≥400 mg·h/liter) was documented in about 12% of cases. A significantly higher proportion of cases with potential overexposure received cotreatment with omeprazole, amiodarone, or amlodipine. Our study suggests that the application of TDM might be especially worthwhile in about 30% of cases with the intent of avoiding either the risk of dose-dependent toxicity or that of treatment failure.
Figures


References
-
- Abunasser, J., and M. L. Metersky. 2009. A comparison of linezolid with glycopeptides in severe MRSA pneumonia. Expert Rev. Anti Infect. Ther. 7:951-955. - PubMed
-
- Adembri, C., S. Fallani, M. I. Cassetta, S. Arrigucci, A. Ottaviano, P. Pecile, T. Mazzei, R. De Gaudio, and A. Novelli. 2008. Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: intermittent versus continuous infusion. Int. J. Antimicrob. Agents 31:122-129. - PubMed
-
- Alffenaar, J. W., J. G. Kosterink, R. V. Altena, T. S. van der Werf, D. R. Uges, and J. H. Proost. 2010. Limited sampling strategies for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Ther. Drug Monit. 32:97-101. - PubMed
-
- Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

