Structure of hibernating ribosomes studied by cryoelectron tomography in vitro and in situ
- PMID: 20733057
- PMCID: PMC2928015
- DOI: 10.1083/jcb.201005007
Structure of hibernating ribosomes studied by cryoelectron tomography in vitro and in situ
Abstract
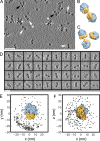
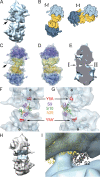
Ribosomes arranged in pairs (100S) have been related with nutritional stress response and are believed to represent a "hibernation state." Several proteins have been identified that are associated with 100S ribosomes but their spatial organization has hitherto not been characterized. We have used cryoelectron tomography to reveal the three-dimensional configuration of 100S ribosomes isolated from starved Escherichia coli cells and we have described their mode of interaction. In situ studies with intact E. coli cells allowed us to demonstrate that 100S ribosomes do exist in vivo and represent an easily reversible state of quiescence; they readily vanish when the growth medium is replenished.
Figures


Similar articles
-
Structure of a hibernating 100S ribosome reveals an inactive conformation of the ribosomal protein S1.Nat Microbiol. 2018 Oct;3(10):1115-1121. doi: 10.1038/s41564-018-0237-0. Epub 2018 Sep 3. Nat Microbiol. 2018. PMID: 30177741
-
The ribosome modulation factor (RMF) binding site on the 100S ribosome of Escherichia coli.J Biochem. 2002 Dec;132(6):983-9. doi: 10.1093/oxfordjournals.jbchem.a003313. J Biochem. 2002. PMID: 12473202
-
Role of HPF (hibernation promoting factor) in translational activity in Escherichia coli.J Biochem. 2008 Mar;143(3):425-33. doi: 10.1093/jb/mvm243. Epub 2008 Jan 2. J Biochem. 2008. PMID: 18174192
-
Stress response as implemented by hibernating ribosomes: a structural overview.FEBS J. 2019 Sep;286(18):3558-3565. doi: 10.1111/febs.14968. Epub 2019 Jul 15. FEBS J. 2019. PMID: 31230411 Free PMC article. Review.
-
Cryo-electron microscopy as an investigative tool: the ribosome as an example.Bioessays. 2001 Aug;23(8):725-32. doi: 10.1002/bies.1102. Bioessays. 2001. PMID: 11494321 Review.
Cited by
-
Absence of Ribosome Modulation Factor Alters Growth and Competitive Fitness of Escherichia coli.Microbiol Spectr. 2022 Apr 27;10(2):e0223921. doi: 10.1128/spectrum.02239-21. Epub 2022 Apr 4. Microbiol Spectr. 2022. PMID: 35377189 Free PMC article.
-
Organization of ribosomes and nucleoids in Escherichia coli cells during growth and in quiescence.J Biol Chem. 2014 Apr 18;289(16):11342-11352. doi: 10.1074/jbc.M114.557348. Epub 2014 Mar 5. J Biol Chem. 2014. PMID: 24599955 Free PMC article.
-
The bacterial translation stress response.FEMS Microbiol Rev. 2014 Nov;38(6):1172-201. doi: 10.1111/1574-6976.12083. Epub 2014 Sep 26. FEMS Microbiol Rev. 2014. PMID: 25135187 Free PMC article. Review.
-
A general mechanism of ribosome dimerization revealed by single-particle cryo-electron microscopy.Nat Commun. 2017 Sep 28;8(1):722. doi: 10.1038/s41467-017-00718-x. Nat Commun. 2017. PMID: 28959045 Free PMC article.
-
Thermal and Nutritional Regulation of Ribosome Hibernation in Staphylococcus aureus.J Bacteriol. 2018 Nov 26;200(24):e00426-18. doi: 10.1128/JB.00426-18. Print 2018 Dec 15. J Bacteriol. 2018. PMID: 30297357 Free PMC article.
References
-
- Fernández J.J., Sanjurjo J.R., Carazo J.M. 1997. A spectral estimation approach to contrast transfer function detection in electron microscopy. Ultramicroscopy. 68:267–295 10.1016/S0304-3991(97)00032-6 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

