Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression
- PMID: 20733059
- PMCID: PMC2928007
- DOI: 10.1083/jcb.201004082
Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression
Abstract
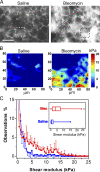
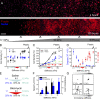
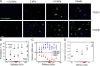
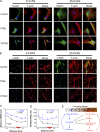
Tissue stiffening is a hallmark of fibrotic disorders but has traditionally been regarded as an outcome of fibrosis, not a contributing factor to pathogenesis. In this study, we show that fibrosis induced by bleomycin injury in the murine lung locally increases median tissue stiffness sixfold relative to normal lung parenchyma. Across this pathophysiological stiffness range, cultured lung fibroblasts transition from a surprisingly quiescent state to progressive increases in proliferation and matrix synthesis, accompanied by coordinated decreases in matrix proteolytic gene expression. Increasing matrix stiffness strongly suppresses fibroblast expression of COX-2 (cyclooxygenase-2) and synthesis of prostaglandin E(2) (PGE(2)), an autocrine inhibitor of fibrogenesis. Exogenous PGE(2) or an agonist of the prostanoid EP2 receptor completely counteracts the proliferative and matrix synthetic effects caused by increased stiffness. Together, these results demonstrate a dominant role for normal tissue compliance, acting in part through autocrine PGE(2), in maintaining fibroblast quiescence and reveal a feedback relationship between matrix stiffening, COX-2 suppression, and fibroblast activation that promotes and amplifies progressive fibrosis.
Figures


References
-
- Bergner A., Sanderson M.J. 2003. Airway contractility and smooth muscle Ca(2+) signaling in lung slices from different mouse strains. J. Appl. Physiol. 95:1325–1332 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

