Distinct 3'UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF)
- PMID: 20733072
- PMCID: PMC2936648
- DOI: 10.1073/pnas.1002929107
Distinct 3'UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF)
Abstract
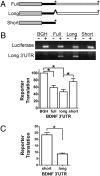
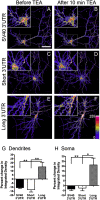
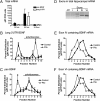
Expression of the brain-derived neurotrophic factor (BDNF) is under tight regulation to accommodate its intricate roles in controlling brain function. Transcription of BDNF initiates from multiple promoters in response to distinct stimulation cues. However, regardless which promoter is used, all BDNF transcripts are processed at two alternative polyadenylation sites, generating two pools of mRNAs that carry either a long or a short 3'UTR, both encoding the same BDNF protein. Whether and how the two distinct 3'UTRs may differentially regulate BDNF translation in response to neuronal activity changes is an intriguing and challenging question. We report here that the long BDNF 3'UTR is a bona fide cis-acting translation suppressor at rest whereas the short 3'UTR mediates active translation to maintain basal levels of BDNF protein production. Upon neuronal activation, the long BDNF 3'UTR, but not the short 3'UTR, imparts rapid and robust activation of translation from a reporter. Importantly, the endogenous long 3'UTR BDNF mRNA specifically undergoes markedly enhanced polyribosome association in the hippocampus in response to pilocarpine induced-seizure before transcriptional up-regulation of BDNF. Furthermore, BDNF protein level is quickly increased in the hippocampus upon seizure-induced neuronal activation, accompanied by a robust activation of the tropomyosin-related receptor tyrosine kinase B. These observations reveal a mechanism for activity-dependent control of BDNF translation and tropomyosin-related receptor tyrosine kinase B signaling in brain neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
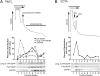
Similar articles
-
HuD promotes BDNF expression in brain neurons via selective stabilization of the BDNF long 3'UTR mRNA.PLoS One. 2013;8(1):e55718. doi: 10.1371/journal.pone.0055718. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383270 Free PMC article.
-
Translational regulation of GluR2 mRNAs in rat hippocampus by alternative 3' untranslated regions.J Neurochem. 2009 Apr;109(2):584-94. doi: 10.1111/j.1471-4159.2009.05992.x. Epub 2009 Feb 13. J Neurochem. 2009. PMID: 19222700 Free PMC article.
-
Brain-derived neurotrophic factor expression is repressed during myogenic differentiation by miR-206.J Neurochem. 2012 Jan;120(2):230-8. doi: 10.1111/j.1471-4159.2011.07583.x. Epub 2011 Dec 6. J Neurochem. 2012. PMID: 22081998
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
-
3'UTR Diversity: Expanding Repertoire of RNA Alterations in Human mRNAs.Mol Cells. 2023 Jan 31;46(1):48-56. doi: 10.14348/molcells.2023.0003. Epub 2023 Jan 20. Mol Cells. 2023. PMID: 36697237 Free PMC article. Review.
Cited by
-
Identification and Characterization of Transcripts Regulated by Circadian Alternative Polyadenylation in Mouse Liver.G3 (Bethesda). 2018 Nov 6;8(11):3539-3548. doi: 10.1534/g3.118.200559. G3 (Bethesda). 2018. PMID: 30181259 Free PMC article.
-
Effects of Genes, Lifestyles, and Noise Kurtosis on Noise-Induced Hearing Loss.Noise Health. 2023 Jul-Sep;25(118):143-157. doi: 10.4103/nah.nah_65_22. Noise Health. 2023. PMID: 37815076 Free PMC article.
-
In vivo interrogation of central nervous system translatome by polyribosome fractionation.J Vis Exp. 2014 Apr 30;(86):51255. doi: 10.3791/51255. J Vis Exp. 2014. PMID: 24835574 Free PMC article.
-
Interaction between miR-142-3p and BDNF Val/Met Polymorphism Regulates Multiple Sclerosis Severity.Int J Mol Sci. 2024 May 11;25(10):5253. doi: 10.3390/ijms25105253. Int J Mol Sci. 2024. PMID: 38791290 Free PMC article.
-
Genetic variability of smoking persistence in African Americans.Cancer Prev Res (Phila). 2011 May;4(5):729-34. doi: 10.1158/1940-6207.CAPR-10-0362. Epub 2011 Mar 24. Cancer Prev Res (Phila). 2011. PMID: 21436384 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD061344/HD/NICHD NIH HHS/United States
- T32 GM008602/GM/NIGMS NIH HHS/United States
- 5T32GM008602/GM/NIGMS NIH HHS/United States
- R01 NS050596/NS/NINDS NIH HHS/United States
- NS036604/NS/NINDS NIH HHS/United States
- NS056532/NS/NINDS NIH HHS/United States
- R01 NS070526/NS/NINDS NIH HHS/United States
- F31 NS056532/NS/NINDS NIH HHS/United States
- R21 NS066235/NS/NINDS NIH HHS/United States
- NS050596/NS/NINDS NIH HHS/United States
- HD061344/HD/NICHD NIH HHS/United States
- R01 NS036604/NS/NINDS NIH HHS/United States
- R56 NS050596/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

