Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3
- PMID: 20733134
- PMCID: PMC4135183
- DOI: 10.1200/JCO.2010.28.9678
Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3
Abstract
Purpose: Mutations leading to constitutive activation of the FMS-like tyrosine kinase 3 receptor (FLT3) occur in blasts of 30% of patients with acute myeloid leukemia (AML). Midostaurin (PKC412; N-benzoylstaurosporin) is a multitargeted tyrosine kinase inhibitor, with demonstrated activity in patients with AML/myelodysplastic syndrome (MDS) with FLT3 mutations.
Patients and methods: Ninety-five patients with AML or MDS with either wild-type (n = 60) or mutated (n = 35) FLT3 were randomly assigned to receive oral midostaurin at 50 or 100 mg twice daily. The drug was discontinued in the absence of response at 2 months, disease progression, or unacceptable toxicity. Response was defined as complete response, partial response (PR), hematologic improvement, or reduction in peripheral blood or bone marrow blasts by ≥ 50% (BR).
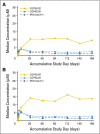
Results: The rate of BR for the population in whom efficacy could be assessed (n = 92) was 71% in patients with FLT3-mutant and 42% in patients with FLT3 wild-type. One PR occurred in a patient with FLT3-mutant receiving the 100-mg dose regimen. Both doses were well-tolerated; there were no differences in toxicity or response rate according to the dose of midostaurin.
Conclusion: These results suggest that midostaurin has hematologic activity in both patients with FLT3-mutant and wild-type. The degree of clinical activity observed supports additional studies that combine midostaurin and other agents such as chemotherapy especially in FLT3-mutant AML.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Burnett AK, Goldstone AH, Stevens RM, et al. Randomised comparison of addition of autologous bone-marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: Results of MRC AML 10 trial: UK Medical Research Council Adult and Children's Leukaemia Working Parties. Lancet. 1998;351:700–708. - PubMed
-
- Stone RM. The difficult problem of acute myeloid leukemia in the older adult. CA Cancer J Clin. 2002;52:363–371. - PubMed
-
- Breitenbuecher F, Schnittger S, Grundler R, et al. Identification of a novel type of ITD mutations located in nonjuxtamembrane domains of the FLT3 tyrosine kinase receptor. Blood. 2009;113:4074–4077. - PubMed
-
- Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2386–2392. - PubMed
-
- Gilliland DG, Griffin JD. Role of FLT3 in leukemia. Curr Opin Hematol. 2002;9:274–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

