Th17 immune responses contribute to the pathophysiology of aplastic anemia
- PMID: 20733158
- PMCID: PMC2993623
- DOI: 10.1182/blood-2010-01-266098
Th17 immune responses contribute to the pathophysiology of aplastic anemia
Abstract
T helper type 17 (Th17) cells have been characterized based on production of interleukin-17 (IL-17) and association with autoimmune diseases. We studied the role of Th17 cells in aplastic anemia (AA) by isolating Th17 cells from patients blood (n = 41) and bone marrow (BM) mononuclear cells (n = 7). The frequency and total number of CD3(+)CD4(+)IL-17-producing T cells were increased in AA patients at presentation compared with healthy controls (P = .0007 and .02, respectively) and correlated with disease activity. There was an inverse relationship between the numbers of Th17 cells and CD4(+)CD25(high)FoxP3(+) regulatory T cells (Tregs) in the blood of AA patients. Concomitant with the classical Th1 response, we detected the presence of CD4(+) and CD8(+) IL-17-producing T cells in a mouse model of lymph node infusion-induced BM failure. Although anti-IL-17 treatment did not abrogate BM failure, early treatment with the anti-IL-17 antibody reduced the severity of BM failure with significantly higher platelet (P < .01) and total BM cell (P < .05) counts at day 10. Recipients that received anti-IL-17 treatment had significantly fewer Th1 cells (P < .01) and more Treg cells (P < .05) at day 10 after lymph node infusion. Th17 immune responses contribute to AA pathophysiology, especially at the early stage during disease progression.
Figures
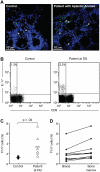



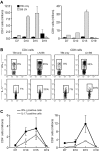


Comment in
-
Abnormalities in Th17 T cells in aplastic anemia.Blood. 2010 Nov 18;116(20):4039-40. doi: 10.1182/blood-2010-09-305367. Blood. 2010. PMID: 21088141 No abstract available.
Similar articles
-
Expression of IL-27, Th1 and Th17 in patients with aplastic anemia.J Clin Immunol. 2013 Feb;33(2):436-45. doi: 10.1007/s10875-012-9810-0. Epub 2012 Sep 30. J Clin Immunol. 2013. PMID: 23054344
-
[Expression of blood Th17 and CD4(+) CD25(+) Treg cells in patients with aplastic anemia].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013 Aug;21(4):974-8. doi: 10.7534/j.issn.1009-2137.2013.04.031. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013. PMID: 23998596 Chinese.
-
Involvement of interleukin-21 in the pathophysiology of aplastic anemia.Eur J Haematol. 2015 Jul;95(1):44-51. doi: 10.1111/ejh.12471. Epub 2015 Mar 12. Eur J Haematol. 2015. PMID: 25784172
-
[Recent progress of study on imbalance of Th17/Treg cells in aplastic anemia].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012 Feb;20(1):214-8. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012. PMID: 22391200 Review. Chinese.
-
Regulatory B and T cell responses in patients with autoimmune thyroid disease and healthy controls.Dan Med J. 2016 Feb;63(2):B5177. Dan Med J. 2016. PMID: 26836805 Review.
Cited by
-
Coptidis alkaloids extracted from Coptis chinensis Franch attenuate IFN-γ-induced destruction of bone marrow cells.PLoS One. 2020 Jul 24;15(7):e0236433. doi: 10.1371/journal.pone.0236433. eCollection 2020. PLoS One. 2020. PMID: 32706801 Free PMC article.
-
New therapeutic approaches for protecting hematopoietic stem cells in aplastic anemia.Immunol Res. 2013 Dec;57(1-3):34-43. doi: 10.1007/s12026-013-8449-0. Immunol Res. 2013. PMID: 24203441 Review.
-
IL-11 promotes the treatment efficacy of hematopoietic stem cell transplant therapy in aplastic anemia model mice through a NF-κB/microRNA-204/thrombopoietin regulatory axis.Exp Mol Med. 2017 Dec 8;49(12):e410. doi: 10.1038/emm.2017.217. Exp Mol Med. 2017. PMID: 29217821 Free PMC article.
-
Expression of IL-27, Th1 and Th17 in patients with aplastic anemia.J Clin Immunol. 2013 Feb;33(2):436-45. doi: 10.1007/s10875-012-9810-0. Epub 2012 Sep 30. J Clin Immunol. 2013. PMID: 23054344
-
Severe aplastic anemia associated with eosinophilic fasciitis: report of 4 cases and review of the literature.Medicine (Baltimore). 2013 Mar;92(2):69-81. doi: 10.1097/MD.0b013e3182899e78. Medicine (Baltimore). 2013. PMID: 23429351 Free PMC article. Review.
References
-
- Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. - PubMed
-
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8(4):345–350. - PubMed
-
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

