Phase I trial of CYT997, a novel cytotoxic and vascular-disrupting agent
- PMID: 20733579
- PMCID: PMC2938266
- DOI: 10.1038/sj.bjc.6605841
Phase I trial of CYT997, a novel cytotoxic and vascular-disrupting agent
Abstract
Background: CYT997 is a novel microtubule inhibitor and vascular-disrupting agent with marked preclinical anti-tumour activity.
Methods: This phase I dose-escalation study assessed the safety, tolerability, pharmacokinetics and pharmacodynamics of CYT997 administered by continuous intravenous infusion over 24 h every 3 weeks to patients with advanced solid tumours.
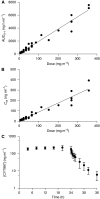
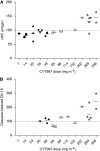
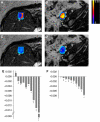
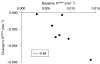
Results: Thirty-one patients received CYT997 over 12 dose levels (7-358 mg m(-2)). Doses up to 202 mg m(-2) were well tolerated. Dose-limiting toxicities were observed at 269 and 358 mg m(-2), consisting of grade 3 prolonged corrected QT interval in two patients and grade 3 hypoxia and grade 4 dyspnea in one patient. All toxicities were reversible. The pharmacokinetics of CYT997 were linear over the entire dose range. Dynamic contrast-enhanced magnetic resonance imaging scans showed significant changes in tumour K(trans) values consistent with vascular disruption in 7 out of 11 evaluable patients treated at CYT997 doses of >or=65 mg m(-2). Moreover, plasma levels of von Willebrand factor and caspase-cleaved cytokeratin-18 increased post-treatment at higher dose levels. Among 22 patients evaluable for response, 18 achieved stable disease for >2 cycles.
Conclusions: CYT997 was well tolerated at doses that were associated with pharmacodynamic evidence of vascular disruption in tumours.
Figures
References
-
- Beerepoot LV, Radema SA, Witteveen EO, Thomas T, Wheeler C, Kempin S, Voest EE (2006) Phase I clinical evaluation of weekly administration of the novel vascular-targeting agent, ZD6126, in patients with solid tumors. J Clin Oncol 24: 1491–1498 - PubMed
-
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS (2006) The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 6: 835–845 - PubMed
-
- Burns CJ, Fantino E, Phillips ID, Su S, Harte MF, Bukczynska PE, Frazzetto M, Joffe M, Kruszelnicki I, Wang B, Wang Y, Wilson N, Dilley RJ, Wan SS, Charman SA, Shackleford DM, Fida R, Malcontenti-Wilson C, Wilks AF (2009a) CYT997: a novel orally active tubulin polymerization inhibitor with potent cytotoxic and vascular disrupting activity in vitro and in vivo. Mol Cancer Ther 8: 3036–3045 - PubMed
-
- Burns CJ, Harte MF, Bu X, Fantino E, Joffe M, Sikanyika H, Su S, Tranberg CE, Wilson N, Charman SA, Shackleford DM, Wilks AF (2009b) Discovery of CYT997: a structurally novel orally active microtubule targeting agent. Bioorg Med Chem Lett 19: 4639–4642 - PubMed
-
- Chu PG, Weiss LM (2002) Keratin expression in human tissues and neoplasms. Histopathology 40: 403–439 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

