Minimally invasive assessment of tumor angiogenesis by fine needle aspiration and flow cytometry
- PMID: 20734228
- PMCID: PMC3237396
- DOI: 10.1007/s10456-010-9182-0
Minimally invasive assessment of tumor angiogenesis by fine needle aspiration and flow cytometry
Abstract
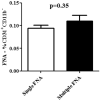
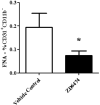
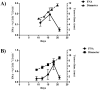
The development of a new, less invasive, and more rapidly implemented method of quantifying endothelial cell density in tumors could facilitate experimental and clinical studies of angiogenesis. Therefore, we evaluated the utility of tumor fine needle aspiration (FNA) coupled with flow cytometry for assessment of tumor angiogenesis. Samples were obtained from cutaneous tumors of mice using FNA, then immunostained and assessed by flow cytometry to determine the number of CD31(+) endothelial cells. Results of the FNA/flow cytometry technique were compared with quantification of tumor microvessel density using immunohistochemistry. The ability of the FNA/cytometry technique to quantify the effects of anti-angiogenic therapy and to monitor changes in tumor angiogenesis over time in individual tumors was also determined. We found that endothelial cell percentages determined in tumor tissue aspirates by flow cytometry correlated well with the percentages of endothelial cells determined in whole tumor digests by flow cytometry and with tumor microvessel density measurements by immunohistochemistry. Moreover, we found that repeated FNA sampling of tumors did not induce endothelial cell changes. Interestingly, by employing repeated FNA sampling of the same tumors we were able to observe a sudden and marked decline in tumor angiogenesis triggered when tumors reached a certain size. Thus, we conclude that the FNA/flow cytometry technique is an efficient, reproducible, and relatively non-invasive method of rapidly assessing tumor angiogenesis, which could be readily applied to evaluation of tumor angiogenesis in clinical settings in humans.
Conflict of interest statement
Figures

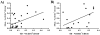

Similar articles
-
In-vivo visualization of tumor microvessel density and response to anti-angiogenic treatment by high resolution MRI in mice.PLoS One. 2011 May 5;6(5):e19592. doi: 10.1371/journal.pone.0019592. PLoS One. 2011. PMID: 21573168 Free PMC article.
-
Repeated fine-needle aspiration of solid tumours in mice allows the identification of multiple infiltrating immune cell types.J Immunol Methods. 2015 Oct;425:102-107. doi: 10.1016/j.jim.2015.06.015. Epub 2015 Jul 6. J Immunol Methods. 2015. PMID: 26159390
-
Flow cytometric analysis of fine needle aspirates is affected by tumor subtype, but not by anatomic location nor technique.Diagn Cytopathol. 2020 Jun;48(6):538-546. doi: 10.1002/dc.24417. Epub 2020 Mar 24. Diagn Cytopathol. 2020. PMID: 32212260
-
Fine-needle aspiration and core needle biopsy: An update on 2 common minimally invasive tissue sampling modalities.Cancer Cytopathol. 2016 Dec;124(12):862-870. doi: 10.1002/cncy.21742. Epub 2016 May 16. Cancer Cytopathol. 2016. PMID: 27182895 Review.
-
Endoscopic/Endobronchial Ultrasound-Guided Fine Needle Aspiration and Ancillary Techniques, Particularly Flow Cytometry, in Diagnosing Deep-Seated Lymphomas.Acta Cytol. 2016;60(4):326-335. doi: 10.1159/000447253. Epub 2016 Jul 15. Acta Cytol. 2016. PMID: 27414717 Review.
Cited by
-
Preclinical evaluation of PSMA expression in response to androgen receptor blockade for theranostics in prostate cancer.EJNMMI Res. 2018 Oct 29;8(1):96. doi: 10.1186/s13550-018-0451-z. EJNMMI Res. 2018. PMID: 30374743 Free PMC article.
-
Fine needle aspiration: an atraumatic method to diagnose head and neck masses.Trauma Mon. 2013 Dec;18(3):117-21. doi: 10.5812/traumamon.10541. Epub 2013 Oct 13. Trauma Mon. 2013. PMID: 24350168 Free PMC article.
-
Novel non-terminal tumor sampling procedure using fine needle aspiration supports immuno-oncology biomarker discovery in preclinical mouse models.J Immunother Cancer. 2021 Jun;9(6):e002894. doi: 10.1136/jitc-2021-002894. J Immunother Cancer. 2021. PMID: 34145033 Free PMC article.
-
Modeling the inhibition of breast cancer growth by GM-CSF.J Theor Biol. 2012 Jun 21;303:141-51. doi: 10.1016/j.jtbi.2012.03.024. Epub 2012 Mar 28. J Theor Biol. 2012. PMID: 22763136 Free PMC article.
-
Depletion of phagocytic myeloid cells triggers spontaneous T cell- and NK cell-dependent antitumor activity.Oncoimmunology. 2012 Nov 1;1(8):1248-1257. doi: 10.4161/onci.21317. Oncoimmunology. 2012. PMID: 23243588 Free PMC article.
References
-
- Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. - PubMed
-
- Folkman J. Tumor angiogenesis: a possible control point in tumor growth. Ann Intern Med. 1975;82:96–100. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Vermeulen PB, Gasparini G, Fox SB, et al. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474–2484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

