The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment
- PMID: 20734454
- PMCID: PMC3179348
- DOI: 10.1002/jbmr.226
The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment
Abstract
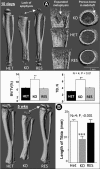
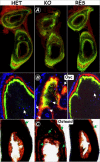
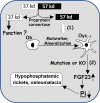
Dentin matrix protein 1 (DMP-1) is a key molecule in controlling osteocyte formation and phosphate homeostasis. Based on observations that full-length DMP-1 is not found in bone, but only cleaved fragments of 37 and 57 kDa are present, and in view of the finding that mutations in the 57-kDa fragment result in disease, we hypothesized that the 57-kDa C-terminal fragment is the functional domain of DMP-1. To test this hypothesis, a 3.6-kb type I collagen promoter was used to express this 57-kDa C-terminal fragment for comparison with full-length DMP-1 in Dmp1 null osteoblasts/osteocytes. Not only did expression of the full-length DMP-1 in bone cells fully rescue the skeletal abnormalities of Dmp1 null mice, but the 57-kDa fragment also had similar results. This included rescue of growth plate defects, osteomalacia, abnormal osteocyte maturation, and the abnormal osteocyte lacunocanalicular system. In addition, the abnormal fibroblast growth factor 23 (FGF-23) expression in osteocytes, elevated circulating FGF-23 levels, and hypophosphatemia were rescued. These results show that the 57-kDa C-terminal fragment is the functional domain of DMP-1 that controls osteocyte maturation and phosphate metabolism.
Copyright © 2011 American Society for Bone and Mineral Research.
Figures




References
-
- George A, Sabsay B, Simonian PA, Veis A. Characterization of a novel dentin matrix acidic phosphoproteimplications for induction of biomineralization. J Biol Chem. 1993;268:12624–12630. - PubMed
-
- Toyosawa S, Shintani S, Fujiwara T, et al. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16:2017–2026. - PubMed
-
- Fen JQ, Zhang J, Dallas SL, et al. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res. 2002;17:1822–1831. 2001. - PubMed
-
- Ye L, MacDougall M, Zhang S, et al. Deletion of dentin matrix protein 1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

