Regulation of catch binding by allosteric transitions
- PMID: 20735005
- PMCID: PMC2938877
- DOI: 10.1021/jp1031459
Regulation of catch binding by allosteric transitions
Abstract
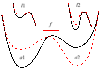
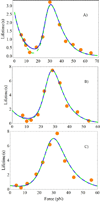
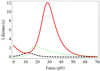
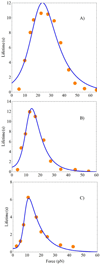
An allosteric model is used to describe changes in lifetimes of biological receptor-ligand bonds subjected to an external force. Force-induced transitions between the two states of the allosteric site lead to changes in the receptor conformation. The ligand bound to the receptor fluctuates between two different potentials formed by the two receptor conformations. The effect of the force on the receptor-ligand interaction potential is described by the Bell mechanism. The probability of detecting the ligand in the bound state is found to depend on the relaxation times of both ligand and allosteric sites. An analytic expression for the bond lifetime is derived as a function of force. The formal theoretical results are used to explain the anomalous force and time dependences of the integrin-fibronectin bond lifetimes measured by atomic force microscopy (Kong, F.; et al J. Cell Biol. 2009, 185, 1275-1284). The analytic expression and model parameters describe very well all anomalous dependences identified in the experiments.
Figures

Similar articles
-
The two-pathway model of the biological catch-bond as a limit of the allosteric model.Biophys J. 2011 Oct 19;101(8):2026-36. doi: 10.1016/j.bpj.2011.09.005. Biophys J. 2011. PMID: 22004757 Free PMC article.
-
Demonstration of catch bonds between an integrin and its ligand.J Cell Biol. 2009 Jun 29;185(7):1275-84. doi: 10.1083/jcb.200810002. J Cell Biol. 2009. PMID: 19564406 Free PMC article.
-
Theoretical aspects of the biological catch bond.Acc Chem Res. 2009 Jun 16;42(6):693-703. doi: 10.1021/ar800202z. Acc Chem Res. 2009. PMID: 19331389 Review.
-
Allosteric role of the large-scale domain opening in biological catch-binding.Phys Rev E Stat Nonlin Soft Matter Phys. 2009 May;79(5 Pt 1):051913. doi: 10.1103/PhysRevE.79.051913. Epub 2009 May 18. Phys Rev E Stat Nonlin Soft Matter Phys. 2009. PMID: 19518486
-
Mechanochemistry of receptor-ligand bonds.Curr Opin Struct Biol. 2009 Feb;19(1):50-5. doi: 10.1016/j.sbi.2008.12.006. Epub 2009 Jan 20. Curr Opin Struct Biol. 2009. PMID: 19157853 Review.
Cited by
-
The two-pathway model of the biological catch-bond as a limit of the allosteric model.Biophys J. 2011 Oct 19;101(8):2026-36. doi: 10.1016/j.bpj.2011.09.005. Biophys J. 2011. PMID: 22004757 Free PMC article.
-
Engineering tunable catch bonds with DNA.Nat Commun. 2024 Oct 12;15(1):8828. doi: 10.1038/s41467-024-52749-w. Nat Commun. 2024. PMID: 39396048 Free PMC article.
-
Mechanochemitry: a molecular biomechanics view of mechanosensing.Ann Biomed Eng. 2014 Feb;42(2):388-404. doi: 10.1007/s10439-013-0904-5. Epub 2013 Sep 5. Ann Biomed Eng. 2014. PMID: 24006131 Free PMC article. Review.
References
-
- Dembo M, Torney DC, Saxman K, Hammer D. Proc. Royal Soc. London Series B-Biol. Sci. 1988;234:55. - PubMed
-
- Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Nature. 2003;423:190. - PubMed
-
- Sarangapani KK, Yago T, Klopocki AG, Lawrence MB, Fieger CB, Rosen SD, McEver RP, Zhu C. J Biol. Chem. 2004;279:2291. - PubMed
-
- Bartolo D, Derenyi I, Ajdari A. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;65:051910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

