Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa
- PMID: 20735240
- PMCID: PMC3204934
- DOI: 10.1086/656282
Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa
Abstract
Background: The World Health Organization (WHO) recommends cough as the trigger for tuberculosis screening in human immunodeficiency virus (HIV)-infected patients, with acid-fast bacillus (AFB) smear as the initial diagnostic test. Our objective was to assess the yield and cost of a more intensive tuberculosis screening in HIV-infected patients starting antiretroviral therapy (ART) in Durban, South Africa.
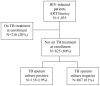
Methods: We prospectively enrolled adults, regardless of tuberculosis signs/symptoms, who were undergoing ART training from May 2007 to May 2008. After the symptom screen, patients expectorated sputum for AFB smear, tuberculosis polymerase chain reaction (PCR), and mycobacterial culture. Sensitivity and specificity of different symptoms and tests, alone and in combination, were compared with the reference standard of 6-week tuberculosis culture results. Program costs included personnel, materials, and cultures.
Results: Of 1035 subjects, 487 (59%) were female; median CD4 cell count was 100 cells/microL. A total of 210 subjects (20%) were receiving tuberculosis treatment and were excluded. Of the remaining 825 subjects, 158 (19%) had positive sputum cultures, of whom 14 (9%) had a positive AFB smear and 82 (52%) reported cough. The combination of cough, other symptoms, AFB smear, and chest radiograph had 93% sensitivity (95% confidence interval, 88%-97%) and 15% specificity (95% confidence interval, 13%-18%). The incremental cost of intensive screening including culture was $360 per additional tuberculosis case identified.
Conclusions: Nearly 20% of patients starting ART in Durban, South Africa, had undiagnosed, culture-positive pulmonary tuberculosis. Despite WHO recommendations, neither cough nor AFB smear were adequately sensitive for screening. Tuberculosis sputum cultures should be performed before ART initiation, regardless of symptoms, in areas with a high prevalence of HIV and tuberculosis.
Conflict of interest statement
There are no conflicts of interest.
Figures
Comment in
-
Undiagnosed active tuberculosis in HIV-infected patients commencing antiretroviral therapy.Clin Infect Dis. 2010 Oct 1;51(7):830-2. doi: 10.1086/656283. Clin Infect Dis. 2010. PMID: 20735239 No abstract available.
-
Tuberculosis screening in patients starting antiretroviral therapy in sub-Saharan Africa: stretching diagnostics to the limits.Clin Infect Dis. 2011 Jan 15;52(2):276-7; author reply 277-8. doi: 10.1093/cid/ciq128. Clin Infect Dis. 2011. PMID: 21288857 Free PMC article. No abstract available.
References
-
- United Nations, Department of Economic and Social Affairs, Population Division. [Accessed 24 December 2009.];World Population Prospects: The 2006 Revision, Highlights, Working Paper No. ESA/P/WP.202. 2007 Available at: http://www.un.org/esa/population/publications/wpp2006/English.pdf.
-
- Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42(7):1040–7. - PubMed
-
- World Health Organization. [Accessed 24 December 2009.];Global tuberculosis control: a short update to the 2009 report. Available at http://www.who.int/tb/publications/global_report/2009/update/tbu_9.pdf.
-
- [Accessed 24 December 2009.];Epidemiological Country Profile on HIV and AIDS: South Africa. 2008 Available at:< http://www.who.int/globalatlas/predefinedReports/EFS2008/short/EFSCountr...>.
-
- Republic of South Africa Department of Health. [Accessed 24 December 2009.];Tuberculosis strategic plan for South Africa: 2007–2011. Available at: http://www.doh.gov.za/docs/index.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

