Lipin proteins form homo- and hetero-oligomers
- PMID: 20735359
- PMCID: PMC3117669
- DOI: 10.1042/BJ20100584
Lipin proteins form homo- and hetero-oligomers
Abstract
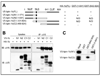
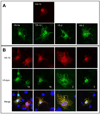
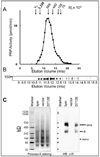
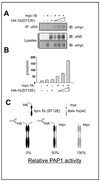
Lipin family members (lipin 1, 2 and 3) are bi-functional proteins that dephosphorylate PA (phosphatidic acid) to produce DAG (diacylglycerol) and act in the nucleus to regulate gene expression. Although other components of the triacylglycerol synthesis pathway can form oligomeric complexes, it is unknown whether lipin proteins also exist as oligomers. In the present study, using various approaches, we revealed that lipin 1 formed stable homo-oligomers with itself and hetero-oligomers with lipin 2/3. Both the N- and C-terminal regions of lipin 1 mediate its oligomerization in a head-to-head/tail-to-tail manner. We also show that lipin 1 subcellular localization can be influenced through oligomerization, and the individual lipin 1 monomers in the oligomer function independently in catalysing dephosphorylation of PA. The present study provides evidence that lipin proteins function as oligomeric complexes and that the three mammalian lipin isoforms can form combinatorial units.
Figures





References
-
- Peterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat. Genet. 2001;27:121–124. - PubMed
-
- Harris TE, Huffman TA, Chi A, Shabanowitz J, Hunt DF, Kumar A, Lawrence JC., Jr. Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J. Biol. Chem. 2007;282:277–286. - PubMed
-
- Peterfy M, Phan J, Reue K. Alternatively spliced lipin isoforms exhibit distinct expression pattern, subcellular localization, and role in adipogenesis. J. Biol. Chem. 2005;280:32883–32889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

