The inhibitor of 20-HETE synthesis, TS-011, improves cerebral microcirculatory autoregulation impaired by middle cerebral artery occlusion in mice
- PMID: 20735406
- PMCID: PMC3000662
- DOI: 10.1111/j.1476-5381.2010.00973.x
The inhibitor of 20-HETE synthesis, TS-011, improves cerebral microcirculatory autoregulation impaired by middle cerebral artery occlusion in mice
Abstract
Background and purpose: 20-Hydroxyeicosatetraenoic acid is a potent vasoconstrictor that contributes to cerebral ischaemia. An inhibitor of 20-Hydroxyeicosatetraenoic acid synthesis, TS-011, reduces infarct volume and improves neurological deficits in animal stroke models. However, little is known about how TS-011 affects the microvessels in ischaemic brain. Here, we investigated the effect of TS-011 on microvessels after cerebral ischaemia.
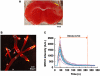
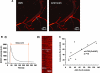
Experimental approach: TS-011 (0.3 mg·kg(-1) ) or a vehicle was infused intravenously for 1 h every 6 h in a mouse model of stroke, induced by transient occlusion of the middle cerebral artery occlusion following photothrombosis. The cerebral blood flow velocity and the vascular perfusion area of the peri-infarct microvessels were measured using in vivo two-photon imaging.
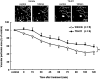
Key results: The cerebral blood flow velocities in the peri-infarct microvessels decreased at 1 and 7 h after reperfusion, followed by an increase at 24 h after reperfusion in the vehicle-treated mice. We found that TS-011 significantly inhibited both the decrease and the increase in the blood flow velocities in the peri-infarct microvessels seen in the vehicle-treated mice after reperfusion. In addition, TS-011 significantly inhibited the reduction in the microvascular perfusion area after reperfusion, compared with the vehicle-treated group. Moreover, TS-011 significantly reduced the infarct volume by 40% at 72 h after middle cerebral artery occlusion.
Conclusions and implications: These findings demonstrated that infusion of TS-011 improved defects in the autoregulation of peri-infarct microcirculation and reduced the infarct volume. Our results could be relevant to the treatment of cerebral ischaemia.
© 2010 The Authors. British Journal of Pharmacology © 2010 The British Pharmacological Society.
Figures


Similar articles
-
Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion.J Cereb Blood Flow Metab. 2009 Mar;29(3):629-39. doi: 10.1038/jcbfm.2008.156. Epub 2008 Dec 24. J Cereb Blood Flow Metab. 2009. PMID: 19107134 Free PMC article.
-
Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N'-hydroxyimido formamide], on hemorrhagic and ischemic stroke.J Pharmacol Exp Ther. 2005 Jul;314(1):77-85. doi: 10.1124/jpet.105.083964. Epub 2005 Apr 14. J Pharmacol Exp Ther. 2005. PMID: 15831442
-
Continuous inhibition of 20-HETE synthesis by TS-011 improves neurological and functional outcomes after transient focal cerebral ischemia in rats.Neurosci Res. 2007 Dec;59(4):475-80. doi: 10.1016/j.neures.2007.08.018. Epub 2007 Sep 6. Neurosci Res. 2007. PMID: 17933409
-
Remodeling of Cerebral Microcirculation after Ischemia-Reperfusion.J Vasc Res. 2015;52(1):22-31. doi: 10.1159/000381096. Epub 2015 Apr 21. J Vasc Res. 2015. PMID: 25896412 Review.
-
The neurovascular unit in the setting of stroke.J Intern Med. 2010 Feb;267(2):156-71. doi: 10.1111/j.1365-2796.2009.02199.x. J Intern Med. 2010. PMID: 20175864 Free PMC article. Review.
Cited by
-
20-HETE Enzymes and Receptors in the Neurovascular Unit: Implications in Cerebrovascular Disease.Front Neurol. 2020 Sep 4;11:983. doi: 10.3389/fneur.2020.00983. eCollection 2020. Front Neurol. 2020. PMID: 33013649 Free PMC article.
-
Conflicting Roles of 20-HETE in Hypertension and Stroke.Int J Mol Sci. 2019 Sep 11;20(18):4500. doi: 10.3390/ijms20184500. Int J Mol Sci. 2019. PMID: 31514409 Free PMC article. Review.
-
Cyclooxygenase- and cytochrome P450-derived eicosanoids in stroke.Prostaglandins Other Lipid Mediat. 2016 Jan;122:45-53. doi: 10.1016/j.prostaglandins.2015.12.007. Epub 2015 Dec 30. Prostaglandins Other Lipid Mediat. 2016. PMID: 26747234 Free PMC article. Review.
-
The contribution of TRPV1 channel to 20-HETE-Aggravated ischemic neuronal injury.Prostaglandins Other Lipid Mediat. 2018 Jul;137:63-68. doi: 10.1016/j.prostaglandins.2018.07.001. Epub 2018 Jul 6. Prostaglandins Other Lipid Mediat. 2018. PMID: 30041768 Free PMC article. Review.
-
The CYP/20-HETE/GPR75 axis in hypertension.Adv Pharmacol. 2022;94:1-25. doi: 10.1016/bs.apha.2022.02.003. Epub 2022 Mar 30. Adv Pharmacol. 2022. PMID: 35659370 Free PMC article. Review.
References
-
- Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke. 1981;12:723–725. - PubMed
-
- Ayata C, Dunn AK, Gursoy-OZdemir Y, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

