GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells
- PMID: 20735417
- PMCID: PMC2931756
- DOI: 10.1111/j.1476-5381.2010.00744.x
GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells
Abstract
Background and purpose: The glycerol-based lysophospholipid lysophosphatidylinositol (LPI) is an endogenous agonist of the G-protein-coupled receptor 55 (GPR55) exhibiting cannabinoid receptor-like properties in endothelial cells. To estimate the contribution of GPR55 to the physiological effects of LPI, the GPR55-dependent and -independent electrical responses in this cell type were investigated.
Experimental approach: Applying small interference RNA-mediated knock-down and transient overexpression, GPR55-dependent and -independent effects of LPI on cytosolic free Ca(2+) concentration, membrane potential and transmembrane ion currents were studied in EA.hy296 cells.
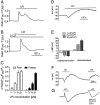
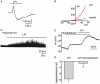
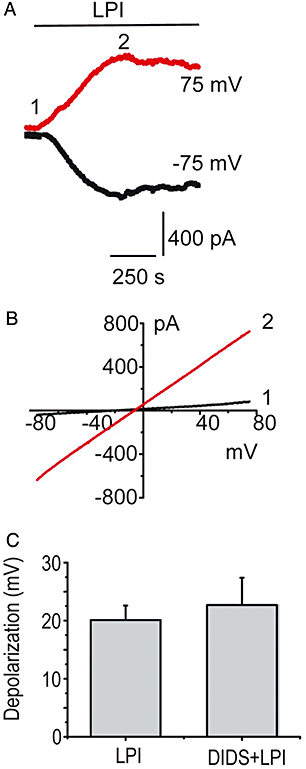
Key results: In a GPR55-dependent, GDPbetaS and U73122-sensitive manner, LPI induced rapid and transient intracellular Ca(2+) release that was associated with activation of charybdotoxin-sensitive, large conductance, Ca(2+)-activated, K(+) channels (BK(Ca)) and temporary membrane hyperpolarization. Following these initial electrical reactions, LPI elicited GPR55-independent long-lasting Na(+) loading and a non-selective inward current causing sustained membrane depolarization that depended on extracellular Ca(2+) and Na(+) and was partially inhibited by Ni(2+) and La(3+). This inward current was due to the activation of a voltage-independent non-selective cation current. The Ni(2+) and La(3+)-insensitive depolarization with LPI was prevented by inhibition of the Na/K-ATPase by ouabain.
Conclusions and implications: LPI elicited a biphasic response in endothelial cells of which the immediate Ca(2+) signalling depends on GPR55 while the subsequent depolarization is due to Na(+) loading via non-selective cation channels and an inhibition of the Na/K-ATPase. Thus, LPI is a potent signalling molecule that affects endothelial functions by modulating several cellular electrical responses that are only partially linked to GPR55.
Figures



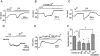

References
-
- Beny JL, Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am J Physiol. 1994;266(4 Pt 2):H1465–H1472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

