Familial hemiplegic migraine Ca(v)2.1 channel mutation R192Q enhances ATP-gated P2X3 receptor activity of mouse sensory ganglion neurons mediating trigeminal pain
- PMID: 20735819
- PMCID: PMC2940876
- DOI: 10.1186/1744-8069-6-48
Familial hemiplegic migraine Ca(v)2.1 channel mutation R192Q enhances ATP-gated P2X3 receptor activity of mouse sensory ganglion neurons mediating trigeminal pain
Abstract
Background: The R192Q mutation of the CACNA1A gene, encoding for the α1 subunit of voltage-gated P/Q Ca2+ channels (Ca(v)2.1), is associated with familial hemiplegic migraine-1. We investigated whether this gain-of-function mutation changed the structure and function of trigeminal neuron P2X3 receptors that are thought to be important contributors to migraine pain.
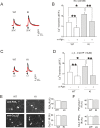
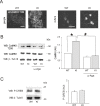
Results: Using in vitro trigeminal sensory neurons of a mouse genetic model knockin for the CACNA1A R192Q mutation, we performed patch clamp recording and intracellular Ca2+ imaging that showed how these knockin ganglion neurons generated P2X3 receptor-mediated responses significantly larger than wt neurons. These enhanced effects were reversed by the Ca(v)2.1 blocker ω-agatoxin. We, thus, explored intracellular signalling dependent on kinases and phosphatases to understand the molecular regulation of P2X3 receptors of knockin neurons. In such cells we observed strong activation of CaMKII reversed by ω-agatoxin treatment. The CaMKII inhibitor KN-93 blocked CaMKII phosphorylation and the hyperesponsive P2X3 phenotype. Although no significant difference in membrane expression of knockin receptors was found, serine phosphorylation of knockin P2X3 receptors was constitutively decreased and restored by KN-93. No change in threonine or tyrosine phosphorylation was detected. Finally, pharmacological inhibitors of the phosphatase calcineurin normalized the enhanced P2X3 receptor responses of knockin neurons and increased their serine phosphorylation.
Conclusions: The present results suggest that the CACNA1A mutation conferred a novel molecular phenotype to P2X3 receptors of trigeminal ganglion neurons via CaMKII-dependent activation of calcineurin that selectively impaired the serine phosphorylation state of such receptors, thus potentiating their effects in transducing trigeminal nociception.
Figures





Similar articles
-
Inefficient constitutive inhibition of P2X3 receptors by brain natriuretic peptide system contributes to sensitization of trigeminal sensory neurons in a genetic mouse model of familial hemiplegic migraine.Mol Pain. 2016 May 12;12:1744806916646110. doi: 10.1177/1744806916646110. Print 2016. Mol Pain. 2016. PMID: 27175010 Free PMC article.
-
Mutated CaV2.1 channels dysregulate CASK/P2X3 signaling in mouse trigeminal sensory neurons of R192Q Cacna1a knock-in mice.Mol Pain. 2013 Dec 2;9:62. doi: 10.1186/1744-8069-9-62. Mol Pain. 2013. PMID: 24294842 Free PMC article.
-
Effects of LPS on P2X3 receptors of trigeminal sensory neurons and macrophages from mice expressing the R192Q Cacna1a gene mutation of familial hemiplegic migraine-1.Purinergic Signal. 2013 Mar;9(1):7-13. doi: 10.1007/s11302-012-9328-1. Epub 2012 Jul 27. Purinergic Signal. 2013. PMID: 22836594 Free PMC article.
-
Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF.Mol Neurobiol. 2008 Feb;37(1):83-90. doi: 10.1007/s12035-008-8020-5. Epub 2008 May 6. Mol Neurobiol. 2008. PMID: 18459072 Review.
-
Role of ATP in migraine mechanisms: focus on P2X3 receptors.J Headache Pain. 2023 Jan 3;24(1):1. doi: 10.1186/s10194-022-01535-4. J Headache Pain. 2023. PMID: 36597043 Free PMC article. Review.
Cited by
-
ATP P2X3 receptors and neuronal sensitization.Front Cell Neurosci. 2013 Dec 4;7:236. doi: 10.3389/fncel.2013.00236. Front Cell Neurosci. 2013. PMID: 24363643 Free PMC article. Review.
-
Brain natriuretic peptide constitutively downregulates P2X3 receptors by controlling their phosphorylation state and membrane localization.Mol Pain. 2015 Nov 14;11:71. doi: 10.1186/s12990-015-0074-6. Mol Pain. 2015. PMID: 26576636 Free PMC article.
-
Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: implications for basic mechanisms of migraine pain.J Neurosci. 2011 Mar 9;31(10):3638-49. doi: 10.1523/JNEUROSCI.6440-10.2011. J Neurosci. 2011. PMID: 21389219 Free PMC article.
-
Animal models of migraine and experimental techniques used to examine trigeminal sensory processing.J Headache Pain. 2019 Aug 29;20(1):91. doi: 10.1186/s10194-019-1043-7. J Headache Pain. 2019. PMID: 31464579 Free PMC article. Review.
-
A Comparative Analysis of the Venom Gland Transcriptomes of the Fishing Spiders Dolomedes mizhoanus and Dolomedes sulfurous.PLoS One. 2015 Oct 7;10(10):e0139908. doi: 10.1371/journal.pone.0139908. eCollection 2015. PLoS One. 2015. PMID: 26445494 Free PMC article.
References
-
- International Headache Society. The International Classification of Headache Disorders, 2nd edition. Cephalalgia. 2004;24:S1–S160. - PubMed
-
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/S0092-8674(00)81373-2. - DOI - PubMed
-
- Tottene A, Conti R, Fabbro A, Vecchia D, Shapovalova M, Santello M, van den Maagdenberg AM, Ferrari MD, Pietrobon D. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in CaV2.1 knockin migraine mice. Neuron. 2009;61:762–773. doi: 10.1016/j.neuron.2009.01.027. - DOI - PubMed
-
- van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, van de Ven RC, Tottene A, van der Kaa J, Plomp JJ, Frants RR, Ferrari MD. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–710. doi: 10.1016/S0896-6273(04)00085-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

