Exploring laccase-like multicopper oxidase genes from the ascomycete Trichoderma reesei: a functional, phylogenetic and evolutionary study
- PMID: 20735824
- PMCID: PMC2939539
- DOI: 10.1186/1471-2091-11-32
Exploring laccase-like multicopper oxidase genes from the ascomycete Trichoderma reesei: a functional, phylogenetic and evolutionary study
Abstract
Background: The diversity and function of ligninolytic genes in soil-inhabiting ascomycetes has not yet been elucidated, despite their possible role in plant litter decay processes. Among ascomycetes, Trichoderma reesei is a model organism of cellulose and hemicellulose degradation, used for its unique secretion ability especially for cellulase production. T. reesei has only been reported as a cellulolytic and hemicellulolytic organism although genome annotation revealed 6 laccase-like multicopper oxidase (LMCO) genes. The purpose of this work was i) to validate the function of a candidate LMCO gene from T. reesei, and ii) to reconstruct LMCO phylogeny and perform evolutionary analysis testing for positive selection.
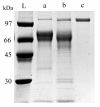
Results: After homologous overproduction of a candidate LMCO gene, extracellular laccase activity was detected when ABTS or SRG were used as substrates, and the recombinant protein was purified to homogeneity followed by biochemical characterization. The recombinant protein, called TrLAC1, has a molecular mass of 104 kDa. Optimal temperature and pH were respectively 40-45°C and 4, by using ABTS as substrate. TrLAC1 showed broad pH stability range of 3 to 7. Temperature stability revealed that TrLAC1 is not a thermostable enzyme, which was also confirmed by unfolding studies monitored by circular dichroism. Evolutionary studies were performed to shed light on the LMCO family, and the phylogenetic tree was reconstructed using maximum-likelihood method. LMCO and classical laccases were clearly divided into two distinct groups. Finally, Darwinian selection was tested, and the results showed that positive selection drove the evolution of sequences leading to well-known laccases involved in ligninolysis. Positively-selected sites were observed that could be used as targets for mutagenesis and functional studies between classical laccases and LMCO from T. reesei.
Conclusions: Homologous production and evolutionary studies of the first LMCO from the biomass-degrading fungus T. reesei gives new insights into the physicochemical parameters and biodiversity in this family.
Figures




Similar articles
-
A novel thermophilic laccase-like multicopper oxidase from Thermothelomyces thermophila and its application in the oxidative cyclization of 2',3,4-trihydroxychalcone.N Biotechnol. 2019 Mar 25;49:10-18. doi: 10.1016/j.nbt.2018.12.001. Epub 2018 Dec 5. N Biotechnol. 2019. PMID: 30529567
-
Expression of Melanocarpus albomyces laccase in Trichoderma reesei and characterization of the purified enzyme.Microbiology (Reading). 2004 Sep;150(Pt 9):3065-3074. doi: 10.1099/mic.0.27147-0. Microbiology (Reading). 2004. PMID: 15347764
-
Mutagenesis of Trichoderma reesei endoglucanase I: impact of expression host on activity and stability at elevated temperatures.BMC Biotechnol. 2015 Feb 21;15(1):11. doi: 10.1186/s12896-015-0118-z. BMC Biotechnol. 2015. PMID: 25879765 Free PMC article.
-
Cellulases and beyond: the first 70 years of the enzyme producer Trichoderma reesei.Microb Cell Fact. 2016 Jun 10;15(1):106. doi: 10.1186/s12934-016-0507-6. Microb Cell Fact. 2016. PMID: 27287427 Free PMC article. Review.
-
The cargo and the transport system: secreted proteins and protein secretion in Trichoderma reesei (Hypocrea jecorina).Microbiology (Reading). 2012 Jan;158(Pt 1):46-57. doi: 10.1099/mic.0.053132-0. Epub 2011 Nov 3. Microbiology (Reading). 2012. PMID: 22053009 Review.
Cited by
-
How Tupanvirus Degrades the Ribosomal RNA of Its Amoebal Host? The Ribonuclease T2 Track.Front Microbiol. 2020 Jul 28;11:1691. doi: 10.3389/fmicb.2020.01691. eCollection 2020. Front Microbiol. 2020. PMID: 32849355 Free PMC article.
-
Whole genome sequencing and analysis of multiple isolates of Ceratocystis destructans, the causal agent of Ceratocystis canker of almond in California.Sci Rep. 2023 Sep 8;13(1):14873. doi: 10.1038/s41598-023-41746-6. Sci Rep. 2023. PMID: 37684350 Free PMC article.
-
Structural and phylogenetic analysis of laccases from Trichoderma: a bioinformatic approach.PLoS One. 2013;8(1):e55295. doi: 10.1371/journal.pone.0055295. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383142 Free PMC article.
-
Pseudomonas-associated bacteria play a key role in obtaining nutrition from bamboo for the giant panda (Ailuropoda melanoleuca).Microbiol Spectr. 2024 Mar 5;12(3):e0381923. doi: 10.1128/spectrum.03819-23. Epub 2024 Feb 2. Microbiol Spectr. 2024. PMID: 38305171 Free PMC article.
-
Fungal laccases and their applications in bioremediation.Enzyme Res. 2014;2014:163242. doi: 10.1155/2014/163242. Epub 2014 May 15. Enzyme Res. 2014. PMID: 24959348 Free PMC article. Review.
References
-
- Martínez AT, Speranza M, Ruiz-Dueñas FJ, Ferreira P, Camarero S, Guillén F, Martínez MJ, Gutiérrez A, del Río JC. Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol. 2005;8:195–204. - PubMed
-
- Levasseur A, Piumi F, Coutinho PM, Rancurel C, Asther M, Delattre M, Henrissat B, Pontarotti P, Asther M, Record E. FOLy: an integrated database for the classification and functional annotation of fungal oxidoreductases potentially involved in the degradation of lignin and related aromatic compounds. Fungal Genet Biol. 2008;45:638–645. doi: 10.1016/j.fgb.2008.01.004. - DOI - PubMed
-
- Thurston CF. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. doi: 10.1099/13500872-140-1-19. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

