Female sex hormones mediate the allergic lung reaction by regulating the release of inflammatory mediators and the expression of lung E-selectin in rats
- PMID: 20735828
- PMCID: PMC2936382
- DOI: 10.1186/1465-9921-11-115
Female sex hormones mediate the allergic lung reaction by regulating the release of inflammatory mediators and the expression of lung E-selectin in rats
Abstract
Background: Fluctuations of estradiol and progesterone levels caused by the menstrual cycle worsen asthma symptoms. Conflicting data are reported in literature regarding pro and anti-inflammatory properties of estradiol and progesterone.
Methods: Female Wistar rats were ovalbumin (OVA) sensitized 1 day after resection of the ovaries (OVx). Control group consisted of sensitized-rats with intact ovaries (Sham-OVx). Allergic challenge was performed by aerosol (OVA 1%, 15 min) two weeks later. Twenty four hours after challenge, BAL, bone marrow and total blood cells were counted. Lung tissues were used as explants, for expontaneous cytokine secretion in vitro or for immunostaining of E-selectin.
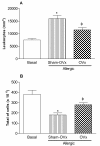
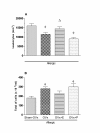
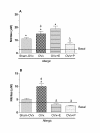
Results: We observed an exacerbated cell recruitment into the lungs of OVx rats, reduced blood leukocytes counting and increased the number of bone marrow cells. Estradiol-treated OVx allergic rats reduced, and those treated with progesterone increased, respectively, the number of cells in the BAL and bone marrow. Lungs of OVx allergic rats significantly increased the E-selectin expression, an effect prevented by estradiol but not by progesterone treatment. Systemically, estradiol treatment increased the number of peripheral blood leukocytes in OVx allergic rats when compared to non treated-OVx allergic rats. Cultured-BAL cells of OVx allergic rats released elevated amounts of LTB4 and nitrites while bone marrow cells increased the release of TNF-alpha and nitrites. Estradiol treatment of OVx allergic rats was associated with a decreased release of TNF-alpha, IL-10, LTB4 and nitrites by bone marrow cells incubates. In contrast, estradiol caused an increase in IL-10 and NO release by cultured-BAL cells. Progesterone significantly increased TNF- alpha by cultured BAL cells and bone marrow cells.
Conclusions: Data presented here suggest that upon hormonal oscillations the immune sensitization might trigger an allergic lung inflammation whose phenotype is under control of estradiol. Our data could contribute to the understanding of the protective role of estradiol in some cases of asthma symptoms in fertile ans post-menopausal women clinically observed.
Figures






References
-
- Frank RT. The hormonal causes of pre-menstrual tension. Arch Neurol Psychiatry. 1931;26:1053–1057.
-
- Pauli BD, Reid RL, Munt PW, Wigle RD, Forkert L. Influence of the menstrual cycle on airway function in asthmatic and normal subjects. Am Rev Respir Dis. 1989;140(2):358–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

