Thymostimulin versus placebo for palliative treatment of locally advanced or metastasised hepatocellular carcinoma: a phase III clinical trial
- PMID: 20735834
- PMCID: PMC2936330
- DOI: 10.1186/1471-2407-10-457
Thymostimulin versus placebo for palliative treatment of locally advanced or metastasised hepatocellular carcinoma: a phase III clinical trial
Abstract
Background: Thymostimulin is a thymic peptide fraction with immune-mediated cytotoxicity against hepatocellular carcinoma (HCC) in vitro and palliative efficacy in advanced HCC in two independent phase II trials. The aim of this study was to assess the efficacy of thymostimulin in a phase III trial.
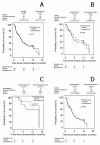
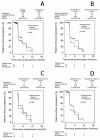
Methods: The study was designed as a prospective randomised, placebo-controlled, double-blind, multicenter clinical phase III trial. Between 10/2002 and 03/2005, 135 patients with locally advanced or metastasised HCC (Karnofsky >or=60%/Child-Pugh <or= 12) were randomised to receive thymostimulin 75 mg s.c. 5x/week or placebo stratified according to liver function. Primary endpoint was twelve-month survival, secondary endpoints overall survival (OS), time to progression (TTP), tumor response, safety and quality of life. A subgroup analysis according to liver function, KPS and tumor stage (Okuda, CLIP and BCLC) formed part of the protocol.
Results: Twelve-month survival was 28% [95%CI 17-41; treatment] and 32% [95%CI 19-44; control] with no significant differences in median OS (5.0 [95% CI 3.7-6.3] vs. 5.2 [95% CI 3.5-6.9] months; p = 0.87, HR = 1.04 [95% CI 0.7-1.6]) or TTP (5.3 [95%CI 2.0-8.6] vs. 2.9 [95%CI 2.6-3.1] months; p = 0.60, HR = 1.13 [95% CI 0.7-1.8]). Adjustment for liver function, Karnofsky status or tumor stage did not affect results. While quality of life was similar in both groups, fewer patients on thymostimulin suffered from accumulating ascites and renal failure.
Conclusions: In our phase III trial, we found no evidence of any benefit to thymostimulin in the treatment of advanced HCC and there is therefore no justification for its use as single-agent treatment. The effect of thymostimulin on hepato-renal function requires further confirmation.
Trial registration: Current Controlled Trials ISRCTN64487365.
Figures


Similar articles
-
Thymostimulin in advanced hepatocellular carcinoma: a phase II trial.BMC Cancer. 2008 Mar 13;8:72. doi: 10.1186/1471-2407-8-72. BMC Cancer. 2008. PMID: 18366627 Free PMC article. Clinical Trial.
-
Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Oncol. 2019 Feb;20(2):282-296. doi: 10.1016/S1470-2045(18)30937-9. Epub 2019 Jan 18. Lancet Oncol. 2019. PMID: 30665869 Clinical Trial.
-
Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial.Lancet Oncol. 2015 Jul;16(7):859-70. doi: 10.1016/S1470-2045(15)00050-9. Epub 2015 Jun 18. Lancet Oncol. 2015. PMID: 26095784 Clinical Trial.
-
Targeting the A3 adenosine receptor to treat hepatocellular carcinoma: anti-cancer and hepatoprotective effects.Purinergic Signal. 2023 Sep;19(3):513-522. doi: 10.1007/s11302-023-09925-2. Epub 2023 Feb 13. Purinergic Signal. 2023. PMID: 36781824 Free PMC article. Review.
-
Indolent cancer and pattern of progression: Two missing parameters in trial design for hepatology.Hepatology. 2024 Jun 1;79(6):1452-1462. doi: 10.1097/HEP.0000000000000527. Epub 2023 Jul 3. Hepatology. 2024. PMID: 37399245 Free PMC article. Review.
Cited by
-
Thymic peptides for treatment of cancer patients.Cochrane Database Syst Rev. 2011 Feb 16;2011(2):CD003993. doi: 10.1002/14651858.CD003993.pub3. Cochrane Database Syst Rev. 2011. PMID: 21328265 Free PMC article.
-
Health-related quality of life in primary hepatic cancer: a systematic review assessing the methodological properties of instruments and a meta-analysis comparing treatment strategies.Qual Life Res. 2021 Sep;30(9):2429-2466. doi: 10.1007/s11136-021-02810-8. Epub 2021 Jul 20. Qual Life Res. 2021. PMID: 34283381 Free PMC article.
-
Quality of life and hepatocellular carcinoma.J Gastrointest Oncol. 2014 Aug;5(4):296-317. doi: 10.3978/j.issn.2078-6891.2014.046. J Gastrointest Oncol. 2014. PMID: 25083303 Free PMC article. Review.
-
Value of quality of life analysis in liver cancer: A clinician's perspective.World J Hepatol. 2017 Jul 18;9(20):867-883. doi: 10.4254/wjh.v9.i20.867. World J Hepatol. 2017. PMID: 28804570 Free PMC article. Review.
-
Evidence-Based Management of Hepatocellular Carcinoma: Systematic Review and Meta-analysis of Randomized Controlled Trials (2002-2020).Gastroenterology. 2021 Sep;161(3):879-898. doi: 10.1053/j.gastro.2021.06.008. Epub 2021 Jun 12. Gastroenterology. 2021. PMID: 34126063 Free PMC article.
References
-
- Fleig WE, Lesske J. Hepatocellular carcinoma: Primary and secondary prophylaxis as well as medical therapy. Chir Gastroenterol. 2003;10:247–252. doi: 10.1159/000074010. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

