PCEP enhances IgA mucosal immune responses in mice following different immunization routes with influenza virus antigens
- PMID: 20735838
- PMCID: PMC2936874
- DOI: 10.1186/1476-8518-8-4
PCEP enhances IgA mucosal immune responses in mice following different immunization routes with influenza virus antigens
Abstract
Background: We previously demonstrated that polyphosphazenes, particularly PCEP, enhance immune responses in mice immunized subcutaneously and intranasally. The objective of the present study was to investigate the efficacy of polyphosphazenes as adjuvants when delivered through different routes of vaccine administration.
Methods: BALB/c mice were immunized through intranasal, subcutaneous, oral and intrarectal delivery with vaccine formulations containing either influenza X:31 antigen alone or formulated in PCEP. Serum and mucosal washes were collected and assayed for antigen-specific antibody responses by ELISA, while splenocytes were assayed for antigen-specific cytokine production by ELISPOT.
Results: Intranasal immunization with PCEP+X:31 induced significantly higher IgA titers in all mucosal secretions (lung, nasal, and vaginal) compared to the other routes. Serum analysis showed that all mice given the PCEP+X:31 combination showed evidence of enhanced IgG2a titers in all administered routes, indicating that PCEP can be effective as an adjuvant in enhancing systemic immune responses when delivered via different routes of administration.
Conclusions: We conclude that PCEP is a potent and versatile mucosal adjuvant that can be administered in a variety of routes and effectively enhances systemic and local immune responses. Furthermore, intranasal immunization was found to be the best administration route for enhancing IgA titers, providing further evidence for the potential of PCEP as a mucosal adjuvant.
Figures

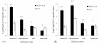
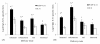

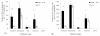
Similar articles
-
Co-administration of polyphosphazenes with CpG oligodeoxynucleotides strongly enhances immune responses in mice immunized with Hepatitis B virus surface antigen.Vaccine. 2008 May 23;26(22):2680-8. doi: 10.1016/j.vaccine.2008.03.031. Epub 2008 Apr 3. Vaccine. 2008. PMID: 18430493
-
Poly[di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP) is a potent enhancer of mixed Th1/Th2 immune responses in mice immunized with influenza virus antigens.Vaccine. 2007 Jan 26;25(7):1204-13. doi: 10.1016/j.vaccine.2006.10.011. Epub 2006 Oct 17. Vaccine. 2007. PMID: 17140708
-
Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration.Vaccine. 2012 Jul 6;30(32):4884-91. doi: 10.1016/j.vaccine.2012.04.032. Epub 2012 Apr 23. Vaccine. 2012. PMID: 22537989
-
Enhanced humoral and mucosal immune responses after intranasal immunization with chimeric multiple antigen peptide of LcrV antigen epitopes of Yersinia pestis coupled to palmitate in mice.Vaccine. 2011 Nov 21;29(50):9352-60. doi: 10.1016/j.vaccine.2011.09.129. Epub 2011 Oct 12. Vaccine. 2011. PMID: 22001881
-
Immunogenicity of Antigen Adjuvanted with AS04 and Its Deposition in the Upper Respiratory Tract after Intranasal Administration.Mol Pharm. 2020 Sep 8;17(9):3259-3269. doi: 10.1021/acs.molpharmaceut.0c00372. Epub 2020 Aug 27. Mol Pharm. 2020. PMID: 32787271
Cited by
-
Nano-Assembled Polyphosphazene Delivery System Enables Effective Intranasal Immunization with Nipah Virus Subunit Vaccine.ACS Appl Bio Mater. 2024 Jun 17;7(6):4133-4141. doi: 10.1021/acsabm.4c00441. Epub 2024 May 30. ACS Appl Bio Mater. 2024. PMID: 38812435 Free PMC article.
-
Polyphosphazenes as Adjuvants for Animal Vaccines and Other Medical Applications.Front Bioeng Biotechnol. 2021 Mar 4;9:625482. doi: 10.3389/fbioe.2021.625482. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33763409 Free PMC article. Review.
-
Future vaccines for a globalized world.Emerg Microbes Infect. 2012 Jul;1(7):e4. doi: 10.1038/emi.2012.17. Emerg Microbes Infect. 2012. PMID: 26038417 Free PMC article. No abstract available.
-
Caspase-1 Dependent IL-1β Secretion and Antigen-Specific T-Cell Activation by the Novel Adjuvant, PCEP.Vaccines (Basel). 2014 Jun 26;2(3):500-14. doi: 10.3390/vaccines2030500. Vaccines (Basel). 2014. PMID: 26344742 Free PMC article.
-
Properties and applications of nanoparticle/microparticle conveyors with adjuvant characteristics suitable for oral vaccination.Int J Nanomedicine. 2018 May 21;13:2973-2987. doi: 10.2147/IJN.S154743. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29861631 Free PMC article. Review.
References
-
- van Ginkel FW, Jackson RJ, Yoshino N, Hagiwara Y, Metzger DJ, Connell TD, Vu HL, Martin M, Fujihashi K, McGhee JR. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect Immun. 2005;73:6892–6902. doi: 10.1128/IAI.73.10.6892-6902.2005. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

