Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children
- PMID: 20735858
- PMCID: PMC2940902
- DOI: 10.1186/1471-2334-10-253
Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children
Abstract
Background: Evidence suggests that probiotics reduce rotavirus diarrhoea duration. Although there are several probiotic strains potentially useful, daily practice is often limited by the type and number of products locally available. In general, information about combined products is scarce. In this study we compare the effect of two probiotic products in the treatment of diarrhoea in children less than 2 years of age.
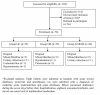
Methods: A Randomized double-blind controlled clinical trial in children hospitalized for acute rotavirus diarrhoea, in the Paediatric Centre Albina Patino, Cochabamba, Bolivia.Participants were children aged 1 - 23 months, who were randomly assigned to receive one of three treatments: Oral rehydration therapy plus placebo; Oral rehydration solution plus Saccharomyces boulardii; or Oral rehydration solution plus a compound containing Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum and Saccharomyces boulardii. Sample size was 20 per group and the outcomes were duration of diarrhoea, of fever, of vomiting and of hospitalization.
Results: 64 cases finished the protocol. On admission, patients' characteristics were similar. Median duration of diarrhoea (p = 0.04) in children who received the single species product (58 hours) was shorter than in controls (84.5 hrs). Comparing children that received the single probiotic product and controls showed shorter duration of fever (18 vs 67 hrs) (p = 0.0042) and the mixed probiotic of vomiting (0 vs 42.5 hrs) (p = 0.041). There was no effect on duration of hospitalization (p = 0.31). When experimental groups were merged, statistical significance of changes increased (total duration of diarrhoea, fever and vomiting P = 0.025, P = 0.025 and P = 0.014, respectively).
Conclusions: Both products decreased the duration of diarrhoea compared to oral rehydration solution alone. This decrease was significant only for the single species product which also decreased the duration of fever. With the multiple species product there was no vomiting subsequent to the initiation of treatment. The quantity of probiotic bacteria needed for optimum treatment of gastroenteritis remains to be determined, particularly when multiple species are included in the product.Trial registration: ClinicalTrials.gov ID: NCT00981877Link: https://register.clinicaltrials.gov/prs/app/action/SelectProtocol/sid/S0002653/selectaction/View/ts/2/uid/U0000N04
Trial registration: Clinical trials NCT ID: NCT00981877.
Figures
References
-
- Panamerican Health Organization. Epidemiologic surveillance of diarrheal diseases due to rotavirus: Field guide. Washington, D.C.: OPS; 2007. (ISBN 92 75 11623 7)
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical