HIV protease inhibitors elicit volume-sensitive Cl- current in cardiac myocytes via mitochondrial ROS
- PMID: 20736017
- PMCID: PMC2988286
- DOI: 10.1016/j.yjmcc.2010.08.013
HIV protease inhibitors elicit volume-sensitive Cl- current in cardiac myocytes via mitochondrial ROS
Abstract
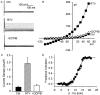
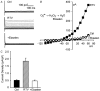
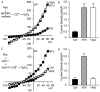
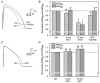
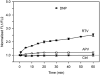
HIV protease inhibitors (HIV PI) reduce morbidity and mortality of HIV infection but cause multiple untoward effects. Because certain HIV PI evoke production of reactive oxygen species (ROS) and volume-sensitive Cl(-) current (I(Cl,swell)) is activated by ROS, we tested whether HIV PI stimulate I(Cl,swell) in ventricular myocytes. Ritonavir and lopinavir elicited outwardly rectifying Cl(-) currents under isosmotic conditions that were abolished by the selective I(Cl,swell)-blocker DCPIB. In contrast, amprenavir, nelfinavir, and raltegravir, an integrase inhibitor, did not modulate I(Cl,swell) acutely. Ritonavir also reduced action potential duration, but amprenavir did not. I(Cl,swell) activation was attributed to ROS because ebselen, an H(2)O(2) scavenger, suppressed ritonavir- and lopinavir-induced I(Cl,swell). Major ROS sources in cardiomyocytes are sarcolemmal NADPH oxidase and mitochondria. The specific NADPH oxidase inhibitor apocynin failed to block ritonavir- or lopinavir-induced currents, although it blocks I(Cl,swell) elicited by osmotic swelling or stretch. In contrast, rotenone, a mitochondrial e(-) transport inhibitor, suppressed both ritonavir- and lopinavir-induced I(Cl,swell). ROS production was measured in HL-1 cardiomyocytes with C-H(2)DCFDA-AM and mitochondrial membrane potential (ΔΨ(m)) with JC-1. Flow cytometry confirmed that ritonavir and lopinavir but not amprenavir, nelfinavir, or raltegravir augmented ROS production, and HIV PI-induced ROS production was suppressed by rotenone but not NADPH oxidase blockade. Moreover, ritonavir, but not amprenavir, depolarized ΔΨ(m). These data suggest ritonavir and lopinavir activated I(Cl,swell) via mitochondrial ROS production that was independent of NADPH oxidase. ROS-dependent modulation of I(Cl,swell) and other ion channels by HIV PI may contribute to some of their actions in heart and perhaps other tissues.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

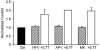

References
-
- Anson BD, Weaver JG, Ackerman MJ, Akinsete O, Henry K, January CT, et al. Blockade of HERG channels by HIV protease inhibitors. Lancet. 2005;365:682–6. - PubMed
-
- Busti AJ, Tsikouris JP, Peeters MJ, Das SR, Canham RM, Abdullah SM, et al. A prospective evaluation of the effect of atazanavir on the QTc interval and QTc dispersion in HIV-positive patients. HIV Med. 2006;7:317–22. - PubMed
-
- Chinello P, Lisena FP, Angeletti C, Boumis E, Papetti F, Petrosillo N. Role of antiretroviral treatment in prolonging QTc interval in HIV-positive patients. J Infect. 2007;54:597–602. - PubMed
-
- Fiala M, Murphy T, MacDougall J, Yang W, Luque A, Iruela-Arispe L, et al. HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovasc Toxicol. 2004;4:327–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

